- Review
- Open access
- Published:
Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review
Respiratory Research volume 23, Article number: 213 (2022)
Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. COPD exacerbations are associated with a worsening of lung function, increased disease burden, and mortality, and, therefore, preventing their occurrence is an important goal of COPD management. This review was conducted to identify the evidence base regarding risk factors and predictors of moderate-to-severe exacerbations in patients with COPD.
Methods
A literature review was performed in Embase, MEDLINE, MEDLINE In-Process, and the Cochrane Central Register of Controlled Trials (CENTRAL). Searches were conducted from January 2015 to July 2019. Eligible publications were peer-reviewed journal articles, published in English, that reported risk factors or predictors for the occurrence of moderate-to-severe exacerbations in adults age ≥ 40 years with a diagnosis of COPD.
Results
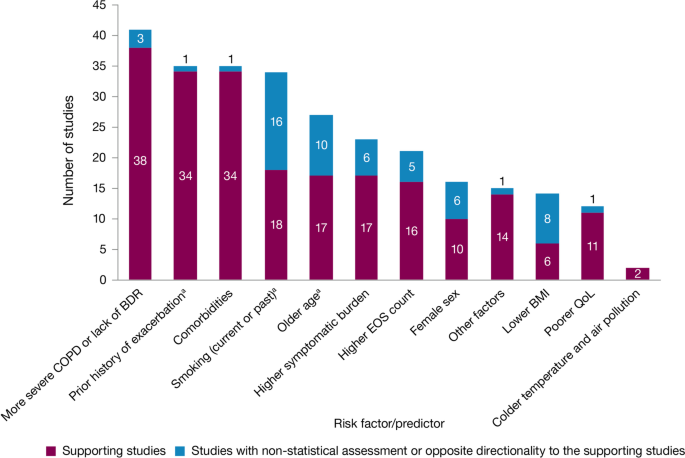
The literature review identified 5112 references, of which 113 publications (reporting results for 76 studies) met the eligibility criteria and were included in the review. Among the 76 studies included, 61 were observational and 15 were randomized controlled clinical trials. Exacerbation history was the strongest predictor of future exacerbations, with 34 studies reporting a significant association between history of exacerbations and risk of future moderate or severe exacerbations. Other significant risk factors identified in multiple studies included disease severity or bronchodilator reversibility (39 studies), comorbidities (34 studies), higher symptom burden (17 studies), and higher blood eosinophil count (16 studies).
Conclusions
This systematic literature review identified several demographic and clinical characteristics that predict the future risk of COPD exacerbations. Prior exacerbation history was confirmed as the most important predictor of future exacerbations. These prognostic factors may help clinicians identify patients at high risk of exacerbations, which are a major driver of the global burden of COPD, including morbidity and mortality.
Background
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide [1]. Based upon disability-adjusted life-years, COPD ranked sixth out of 369 causes of global disease burden in 2019 [2]. COPD exacerbations are associated with a worsening of lung function, and increased disease burden and mortality (of those patients hospitalized for the first time with an exacerbation, > 20% die within 1 year of being discharged) [3]. Furthermore, patients with COPD consider exacerbations or hospitalization due to exacerbations to be the most important disease outcome, having a large impact on their lives [4]. Therefore, reducing the future risk of COPD exacerbations is a key goal of COPD management [5].
Being able to predict the level of risk for each patient allows clinicians to adapt treatment and patients to adjust their lifestyle (e.g., through a smoking cessation program) to prevent exacerbations [3]. As such, identifying high-risk patients using measurable risk factors and predictors that correlate with exacerbations is critical to reduce the burden of disease and prevent a cycle of decline encompassing irreversible lung damage, worsening quality of life (QoL), increasing disease burden, high healthcare costs, and early death.
Prior history of exacerbations is generally thought to be the best predictor of future exacerbations; however, there is a growing body of evidence suggesting other demographic and clinical characteristics, including symptom burden, airflow obstruction, comorbidities, and inflammatory biomarkers, also influence risk [6,7,8,9]. For example, in the prospective ECLIPSE observational study, the likelihood of patients experiencing an exacerbation within 1 year of follow-up increased significantly depending upon several factors, including prior exacerbation history, forced expiratory volume in 1 s (FEV1), St. George’s Respiratory Questionnaire (SGRQ) score, gastroesophageal reflux, and white blood cell count [9].
Many studies have assessed predictors of COPD exacerbations across a variety of countries and patient populations. This systematic literature review (SLR) was conducted to identify and compile the evidence base regarding risk factors and predictors of moderate-to-severe exacerbations in patients with COPD.
Methods
Systematic literature review
A comprehensive search strategy was designed to identify English-language studies published in peer-reviewed journals providing data on risk factors or predictors of moderate or severe exacerbations in adults aged ≥ 40 years with a diagnosis of COPD (sample size ≥ 100). The protocol is summarized in Table 1 and the search strategy is listed in Additional file 1: Table S1. Key biomedical electronic literature databases were searched from January 2015 until July 2019. Other sources were identified via bibliographic searching of relevant systematic reviews.
Study selection process
Implementation and reporting followed the recommendations and standards of the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement [10]. An independent reviewer conducted the first screening based on titles and abstracts, and a second reviewer performed a quality check of the excluded evidence. A single independent reviewer also conducted the second screening based on full-text articles, with a quality check of excluded evidence performed by a second reviewer. Likewise, data tables of the included studies were generated by one reviewer, and another reviewer performed a quality check of extracted data. Where more than one publication was identified describing a single study or trial, data were compiled into a single entry in the data-extraction table to avoid double counting of patients and studies. One publication was designated as the ‘primary publication’ for the purposes of the SLR, based on the following criteria: most recently published evidence and/or the article that presented the majority of data (e.g., journal articles were preferred over conference abstracts; articles that reported results for the full population were preferred over later articles providing results of subpopulations). Other publications reporting results from the same study were designated as ‘linked publications’; any additional data in the linked publications that were not included in the primary publication were captured in the SLR. Conference abstracts were excluded from the SLR unless they were a ‘linked publication.’
Results
Included studies
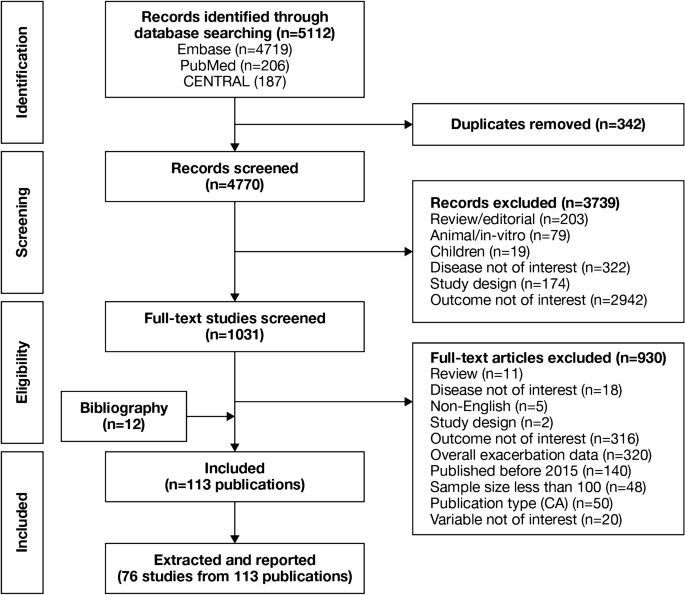
A total of 5112 references (Fig. 1) were identified from the database searches. In total, 76 studies from 113 publications were included in the review. Primary publications and ‘linked publications’ for each study are detailed in Additional file 1: Table S2, and study characteristics are shown in Additional file 1: Table S3. The studies included clinical trials, registry studies, cross-sectional studies, cohort studies, database studies, and case–control studies. All 76 included studies were published in peer-reviewed journals. Regarding study design, 61 of the studies were observational (34 retrospective observational studies, 19 prospective observational studies, four cross-sectional studies, two studies with both retrospective and prospective cohort data, one case–control study, and one with cross-sectional and longitudinal data) and 15 were randomized controlled clinical trials.
Of the 76 studies, 16 were conducted in North America (13 studies in the USA, two in Canada, and one in Mexico); 26 were conducted in Europe (seven studies in Spain, four in the UK, three in Denmark, two studies each in Bulgaria, the Netherlands, and Switzerland, and one study each in Sweden, Serbia, Portugal, Greece, Germany, and France) and 17 were conducted in Asia (six studies in South Korea, four in China, three in Taiwan, two in Japan, and one study each in Singapore and Israel). One study each was conducted in Turkey and Australia. Fifteen studies were conducted across multiple countries.
The majority of the studies (n = 54) were conducted in a multicenter setting, while 22 studies were conducted in a single-center setting. The sample size among the included studies varied from 118 to 339,389 patients.
Patient characteristics
A total of 75 studies reported patient characteristics (Additional file 1: Table S4). The mean age was reported in 65 studies and ranged from 58.0 to 75.2 years. The proportion of male patients ranged from 39.7 to 97.6%. The majority of included studies (85.3%) had a higher proportion of males than females.
Exacerbation history (as defined per each study) was reported in 18 of 76 included studies. The proportion of patients with no prior exacerbation was reported in ten studies (range, 0.1–79.5% of patients), one or fewer prior exacerbation in ten studies (range, 46–100%), one or more prior exacerbation in eight studies (range, 18.4–100%), and two or more prior exacerbations in 12 studies (range, 6.1–55.0%).
Prognostic factors of exacerbations
A summary of the risk factors and predictors reported across the included studies is provided in Tables 2 and 3. The overall findings of the SLR are summarized in Figs. 2 and 3.
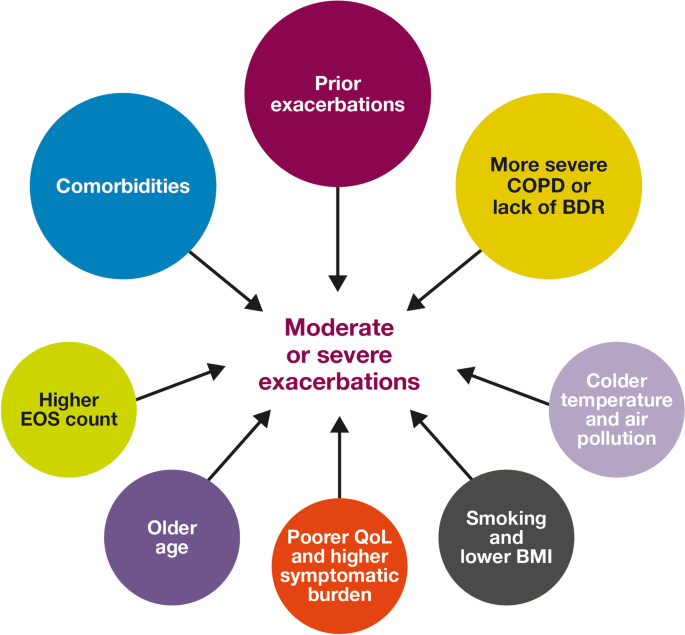
Risk factors for moderate-to-severe exacerbations in patients with COPD. Factors with > 30 supporting studies shown as large circles; factors with ≤ 30 supporting studies shown as small circles and should be interpreted cautiously. BDR bronchodilator reversibility, BMI body mass index, COPD chronic obstructive pulmonary disease, EOS eosinophil, QoL quality of life
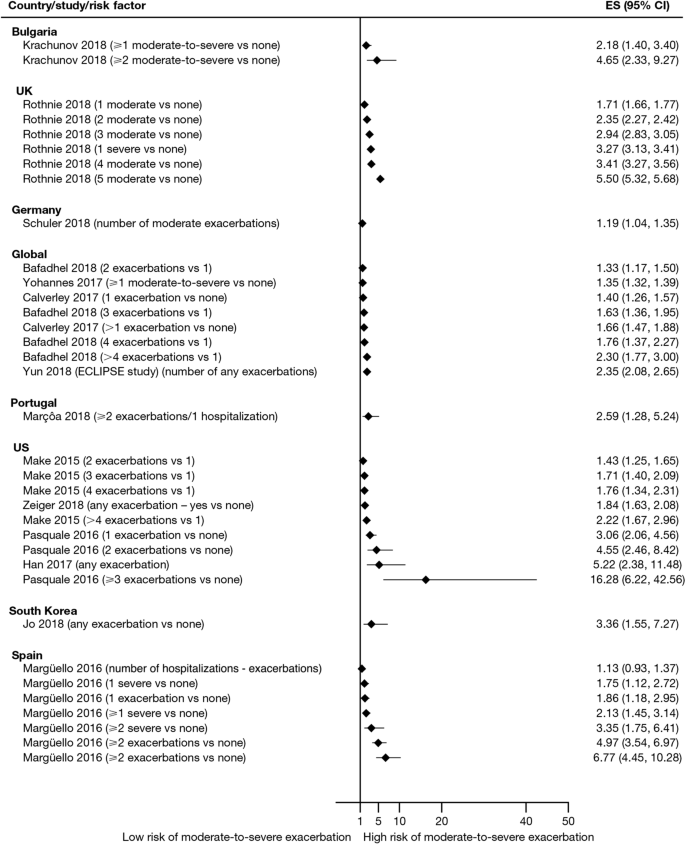
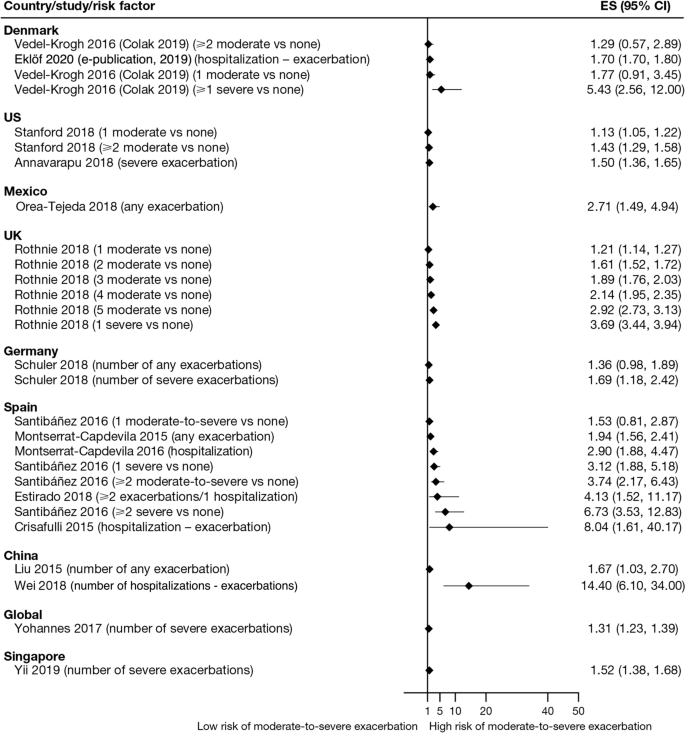
Exacerbation history within the past 12 months was the strongest predictor of future exacerbations. Across the studies assessing this predictor, 34 out of 35 studies (97.1%) reported a significant association between history of exacerbations and risk of future moderate-to-severe exacerbations (Table 3). Specifically, two or more exacerbations in the previous year or at least one hospitalization for COPD in the previous year were identified as reliable predictors of future moderate or severe exacerbations. Even one moderate exacerbation increased the risk of a future exacerbation, with the risk increasing further with each subsequent exacerbation (Fig. 4). A severe exacerbation was also found to increase the risk of subsequent exacerbation and hospitalization (Fig. 5). Patients experiencing one or more severe exacerbations were more likely to experience further severe exacerbations than moderate exacerbations [11, 12]. In contrast, patients with a history of one or more moderate exacerbations were more likely to experience further moderate exacerbations than severe exacerbations [11, 12].
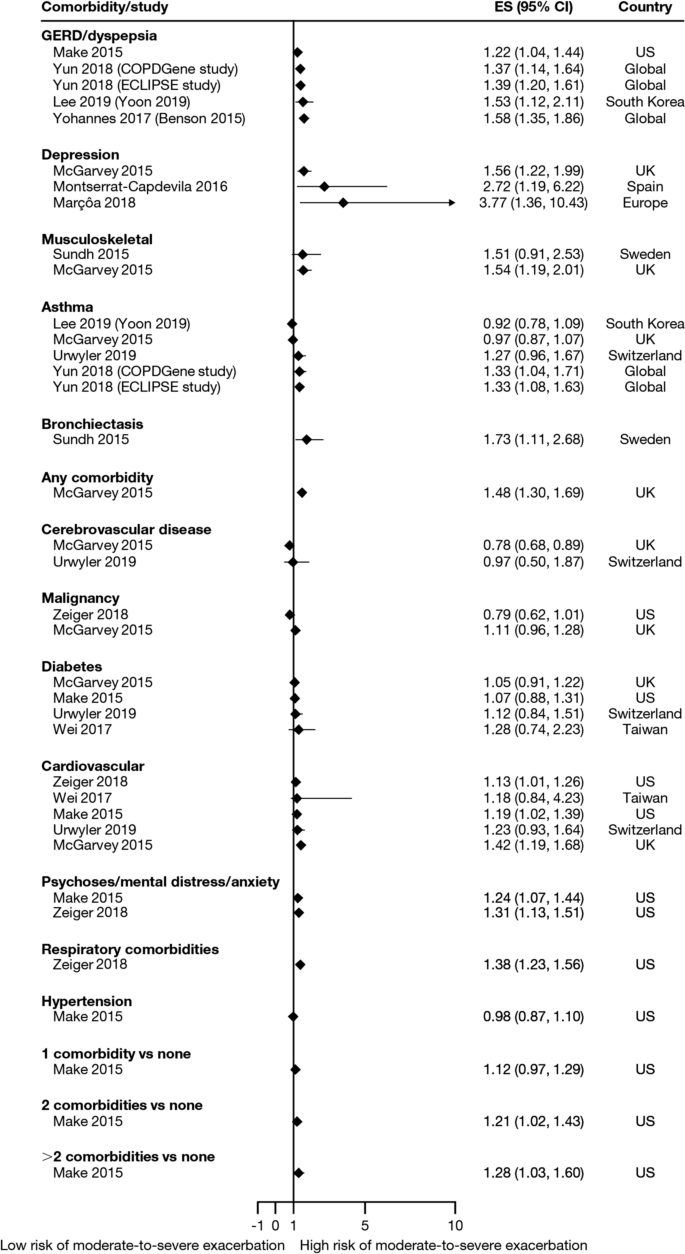
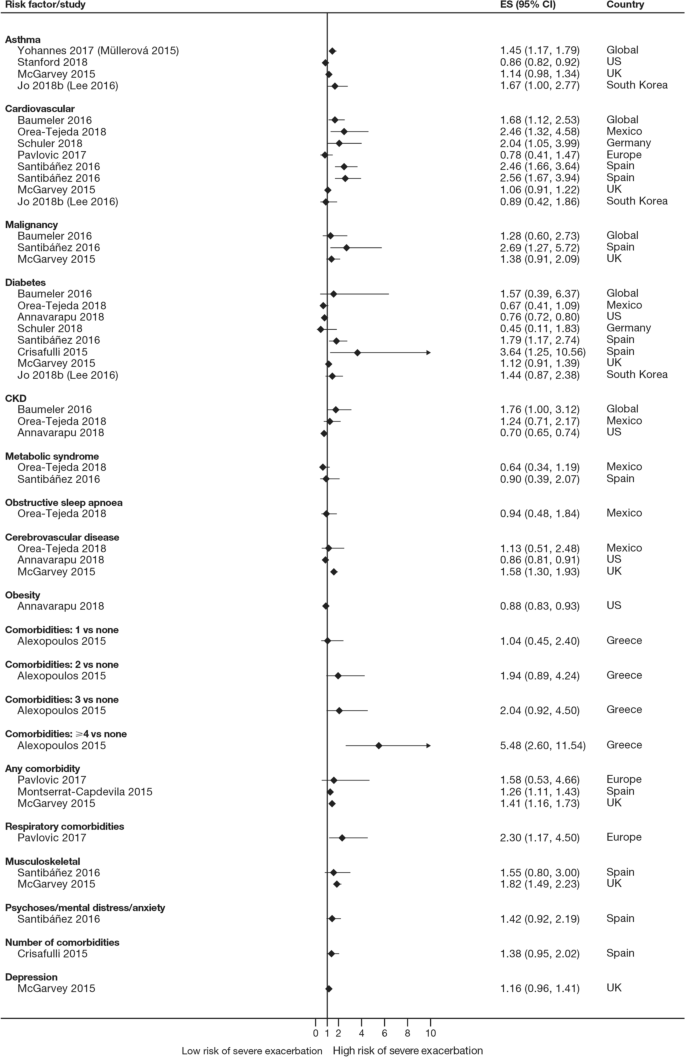
Overall, 35 studies assessed the association of comorbidities with the risk of exacerbation. All studies except one (97.1%) reported a positive association between comorbidities and the occurrence of moderate-to-severe exacerbations (Table 3). In addition to the presence of any comorbidity, specific comorbidities that were found to significantly increase the risk of moderate-to-severe exacerbations included anxiety and depression, cardiovascular comorbidities, gastroesophageal reflux disease/dyspepsia, and respiratory comorbidities (Fig. 6). Comorbidities that were significant risk factors for severe exacerbations included cardiovascular, musculoskeletal, and respiratory comorbidities, diabetes, and malignancy (Fig. 7). Overall, the strongest association between comorbidities and COPD readmissions in the emergency department was with cardiovascular disease. The degree of risk for both moderate-to-severe and severe exacerbations also increased with the number of comorbidities. A Dutch cohort study found that 88% of patients with COPD had at least one comorbidity, with hypertension (35%) and coronary heart disease (19%) being the most prevalent. In this cohort, the comorbidities with the greatest risk of frequent exacerbations were pulmonary cancer (odds ratio [OR] 1.85) and heart failure (OR 1.72) [7].
Comorbidities as risk factors for moderate-to-severe exacerbations. Yun 2018 included two studies; the study from which data were extracted (COPDGene or ECLIPSE) is listed in parentheses. Where data have been extracted from a linked publication rather than the primary publication, the linked publication is listed in parentheses. CI confidence interval, ES effect size, GERD gastroesophageal disease
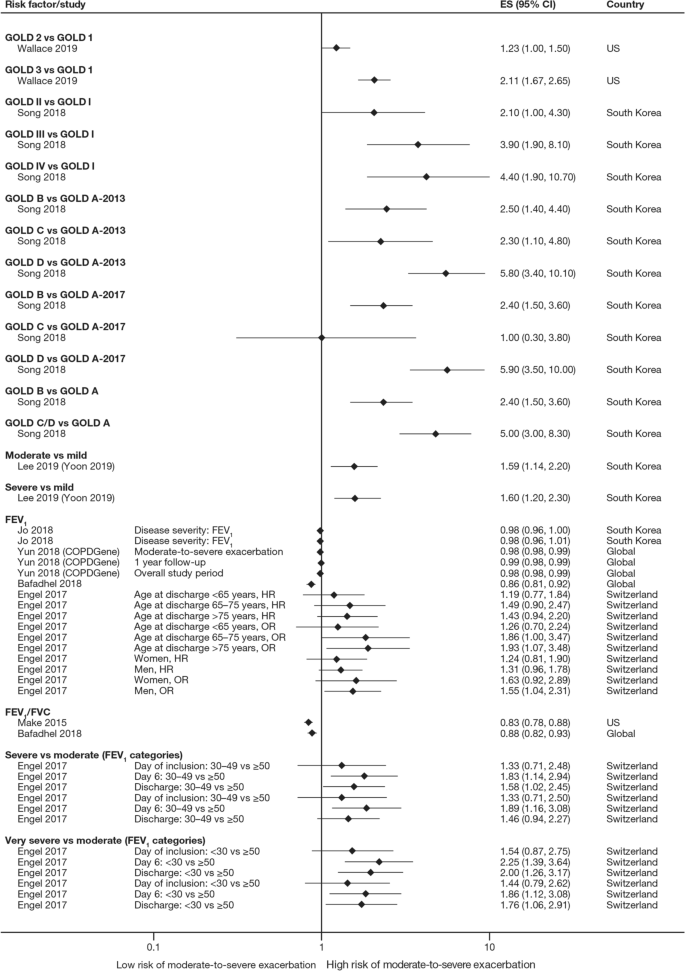
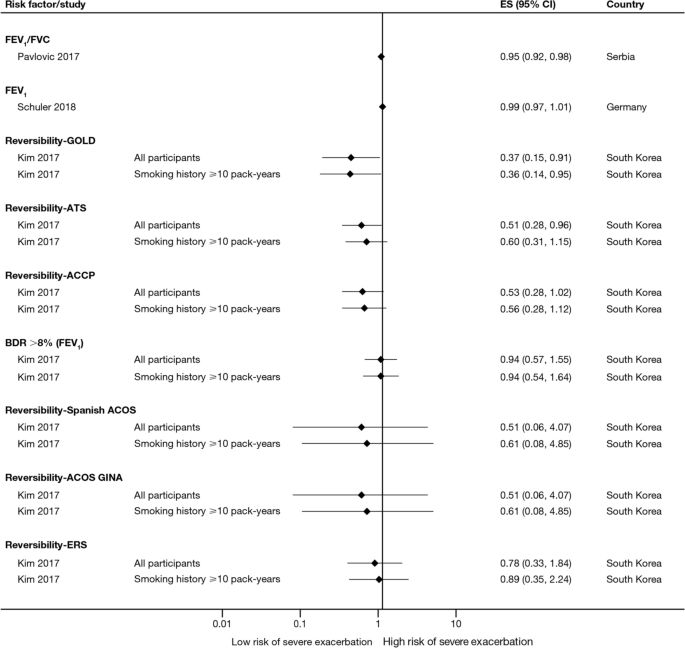
The majority of studies assessing disease severity or bronchodilator reversibility (39/41; 95.1%) indicated a significant positive relation between risk of future exacerbations and greater disease severity, as assessed by greater lung function impairment (in terms of lower FEV1, FEV1/forced vital capacity ratio, or forced expiratory flow [25–75]/forced vital capacity ratio) or more severe Global Initiative for Chronic Obstructive Lung Disease (GOLD) class A − D, and a positive relationship between risk of future exacerbations and lack of bronchodilator reversibility (Table 3, Figs. 8 and 9).
Disease severity as a risk factor for moderate-to-severe exacerbations. Yun 2018 included two studies; the study from which data were extracted (COPDGene or ECLIPSE) is listed in parentheses. Where data have been extracted from a linked publication rather than the primary publication, the linked publication is listed in parentheses. CI confidence interval, ES effect size, FEV1 forced expiratory volume in 1 s, FVC, forced vital capacity, GOLD Global Initiative for Obstructive Lung Disease, HR hazard ratio, OR odds ratio
Disease severity and BDR as risk factors for severe exacerbations. ACCP American College of Chest Physicians, ACOS Asthma-COPD overlap syndrome, ATS American Thoracic Society, BDR bronchodilator reversibility, CI confidence interval, ERS European Respiratory Society, ES effect size, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, GINA Global Initiative for Asthma, GOLD Global Initiative for Obstructive Lung Disease
Of 21 studies assessing the relationship between blood eosinophil count and exacerbations (Table 3), 16 reported estimates for the risk of moderate or severe exacerbations by eosinophil count. A positive association was observed between higher eosinophil count and a higher risk of moderate or severe exacerbations, particularly in patients not treated with an inhaled corticosteroid (ICS); however, five studies reported a significant positive association irrespective of intervention effects. The risk of moderate-to-severe exacerbations was observed to be positively associated with various definitions of higher eosinophil levels (absolute counts: ≥ 200, ≥ 300, ≥ 340, ≥ 400, and ≥ 500 cells/mm3; % of blood eosinophil count: ≥ 2%, ≥ 3%, ≥ 4%, and ≥ 5%). Of note, one study found reduced efficacy of ICS in lowering moderate-to-severe exacerbation rates for current smokers versus former smokers at all eosinophil levels [13].
Of 12 studies assessing QoL scales, 11 (91.7%) studies reported a significant association between the worsening of QoL scores and the risk of future exacerbations (Table 3). Baseline SGRQ [14, 15], Center for Epidemiologic Studies Depression Scale (for which increased scores may indicate impaired QoL) [16], and Clinical COPD Questionnaire [17, 18] scores were found to be associated with future risk of moderate and/or severe COPD exacerbations. For symptom scores, six out of eight studies assessing the association between moderate-to-severe or severe exacerbations with COPD Assessment Test (CAT) scores reported a significant and positive relationship. Furthermore, the risk of moderate-to-severe exacerbations was found to be significantly higher in patients with higher CAT scores (≥ 10) [15, 19,20,21], with one study demonstrating that a CAT score of 15 increased predictive ability for exacerbations compared with a score of 10 or more [18]. Among 15 studies that assessed the association of modified Medical Research Council (mMRC) scores with the risk of moderate-to-severe or severe exacerbation, 11 found that the risk of moderate-to-severe or severe exacerbations was significantly associated with higher mMRC scores (≥ 2) versus lower scores. Furthermore, morning and night symptoms (measured by Clinical COPD Questionnaire) were associated with poor health status and predicted future exacerbations [17].
Of 36 studies reporting the relationship between smoking status and moderate-to-severe or severe exacerbations, 22 studies (61.1%) reported a significant positive association (Table 3). Passive smoking was also significantly associated with an increased risk of severe exacerbations (OR 1.49) [20]. Of note, three studies reported a significantly lower rate of moderate-to-severe exacerbations in current smokers compared with former smokers [22,23,24].
A total of 14 studies assessed the association of body mass index (BMI) with the occurrence of frequent moderate-to-severe exacerbations in patients with COPD. Six out of 14 studies (42.9%) reported a significant negative association between exacerbations and BMI (Table 3). The risk of moderate and/or severe COPD exacerbations was highest among underweight patients compared with normal and overweight patients [23, 25,26,27,28].
In the 29 studies reporting an association between age and moderate or severe exacerbations, more than half found an association of older age with an increased risk of moderate-to-severe exacerbations (58.6%; Table 3). Four of these studies noted a significant increase in the risk of moderate-to-severe or severe exacerbations for every 10-year increase in age [25, 26, 29, 30]. However, 12 studies reported no significant association between age and moderate-to-severe or severe exacerbation risk.
Sixteen out of 33 studies investigating the impact of sex on exacerbation risk found a significant association (48.5%; Table 3). Among these, ten studies reported that female sex was associated with an increased risk of moderate-to-severe exacerbations, while six studies showed a higher exacerbation risk in males compared with females. There was some variation in findings by geographic location and exacerbation severity (Additional file 2: Figs. S1 and S2). Notably, when assessing the risk of severe exacerbations, more studies found an association with male sex compared with female sex (6/13 studies vs 1/13 studies, respectively).
Both studies evaluating associations between exacerbations and environmental factors reported that colder temperature and exposure to major air pollution (NO2, O3, CO, and/or particulate matter ≤ 10 μm in diameter) increased hospital admissions due to severe exacerbations and moderate-to-severe exacerbation rates [31, 32].
Four studies assessed the association of 6-min walk distance with the occurrence of frequent moderate-to-severe exacerbations (Table 3). One study (25.0%) found that shorter 6-min walk distance (representing low physical activity) was significantly associated with a shortened time to severe exacerbation, but the effect size was small (hazard ratio 0.99) [33].
Five out of six studies assessing the relationship between race or ethnicity and exacerbation risk reported significant associations (Table 3). Additionally, one study reported an association between geographic location in the US and exacerbations, with living in the Northeast region being the strongest predictor of severe COPD exacerbations versus living in the Midwest and South regions [34].
Overall, seven studies assessed the association of biomarkers with risk of future exacerbations (Table 3), with the majority identifying significant associations between inflammatory biomarkers and increased exacerbation risk, including higher C-reactive protein levels [8, 35], fibrinogen levels [8, 30], and white blood cell count [8, 15, 16].
Discussion
This SLR has identified several demographic and clinical characteristics that predict the future risk of COPD exacerbations. Key factors associated with an increased risk of future moderate-to-severe exacerbations included a history of prior exacerbations, worse disease severity and bronchodilator reversibility, the presence of comorbidities, a higher eosinophil count, and older age (Fig. 2). These prognostic factors may help clinicians identify patients at high risk of exacerbations, which are a major driver of the burden of COPD, including morbidity and mortality [36].
Findings from this review summarize the existing evidence, validating the previously published literature [6, 9, 23] and suggesting that the best predictor of future exacerbations is a history of exacerbations in the prior year [8, 11,12,13,14, 16,17,18,19,20,21,22,23, 26, 29, 34, 35, 37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. In addition, the effect size generally increased with the number of prior exacerbations, with a stronger effect observed with prior severe versus moderate exacerbations. This effect was observed across regions, including in Europe and North America, and in several global studies. This relationship represents a vicious circle, whereby one exacerbation predisposes a patient to experience future exacerbations and leading to an ever-increasing disease burden, and emphasizes the importance of preventing the first exacerbation event through early, proactive exacerbation prevention. The finding that prior exacerbations tended to be associated with future exacerbations of the same severity suggests that the severity of the underlying disease may influence exacerbation severity. However, the validity of the traditional classification of exacerbation severity has recently been challenged [61], and further work is required to understand relationships with objective assessments of exacerbation severity.
In addition to exacerbation history, disease severity and bronchodilator reversibility were also strong predictors for future exacerbations [8, 14, 16, 18,19,20, 22,23,24, 26, 28, 29, 33, 37, 40, 43,44,45,46, 48, 50,51,52, 56, 59, 62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78]. The association with disease severity was noted in studies that used GOLD disease stages 1–4 and those that used FEV1 percent predicted and other lung function assessments as continuous variables. Again, this risk factor is self-perpetuating, as evidence shows that even a single moderate or severe exacerbation may almost double the rate of lung function decline [79]. Accordingly, disease severity and exacerbation history may be correlated. Margüello et al. concluded that the severity of COPD could be associated with a higher risk of exacerbations, but this effect was partly determined by the exacerbations suffered in the previous year [23]. It should be noted that FEV1 is not recommended by GOLD for use as a predictor of exacerbation risk or mortality alone due to insufficient precision when used at the individual patient level [5].
Another factor that should be considered when assessing individual exacerbation risk is the presence of comorbidities [7, 14, 16, 18,19,20,21,22, 24,25,26,27,28, 30, 33,34,35, 40, 41, 44,45,46,47,48, 51,52,53,54, 56, 58, 59, 63, 64, 73, 74, 76, 77, 80,81,82,83,84,85]. Comorbidities are common in COPD, in part due to common risk factors (e.g., age, smoking, lifestyle factors) that also increase the risk of other chronic diseases [7]. Significant associations were observed between exacerbation risk and comorbidities, such as anxiety and depression, cardiovascular disease, diabetes, and respiratory comorbidities. As with prior exacerbations, the strength of the association increased with the number of comorbidities. Some comorbidities that were found to be associated with COPD exacerbations share a common biological mechanism of systemic inflammation, such as cardiovascular disease, diabetes, and depression [86]. Furthermore, other respiratory comorbidities, including asthma and bronchiectasis, involve inflammation of the airways [87]. In these patients, optimal management of comorbidities may reduce the risk of future COPD exacerbations (and improve QoL), although further research is needed to confirm the efficacy of this approach to exacerbation prevention. As cardiovascular conditions, including hypertension and coronary heart disease, are the most common comorbidities in people with COPD [7], reducing cardiovascular risk may be a key goal in reducing the occurrence of exacerbations. For other comorbidities, the mechanism for the association with exacerbation risk may be related to non-biological factors. For example, in depression, it has been suggested that the mechanism may relate to greater sensitivity to symptom changes or more frequent physician visits [88].
There is now a growing body of evidence reporting the relationship between blood eosinophil count and exacerbation risk [8, 13, 14, 20, 37, 48, 52, 56, 59, 60, 62, 89,90,91,92,93,94,95,96,97,98,99]. Data from many large clinical trials (SUNSET [89], FLAME [96], WISDOM [98], IMPACT [13], TRISTAN [99], INSPIRE [99], KRONOS [91], TRIBUTE [48], TRILOGY [52], TRINITY [56]) have also shown relationships between treatment, eosinophil count, and exacerbation rates. Evidence shows that eosinophil count, along with other effect modifiers (e.g., exacerbation history), can be used to predict reductions in exacerbations with ICS treatment. Identifying patients most likely to respond to ICS should contribute to personalized medicine approaches to treat COPD. One challenge in drawing a strong conclusion from eosinophil counts is the choice of a cut-off value, with a variety of absolute and percentage values observed to be positively associated with the risk of moderate-to-severe exacerbations. The use of absolute counts may be more practical, as these are not affected by variations in other immune cell numbers; however, there is a lack of consensus on this point [100].
Across the studies examined, associations between sex and the risk of moderate and/or severe exacerbations were variable [14, 16, 18, 20,21,22,23,24, 26,27,28,29, 37, 40, 42, 44,45,46,47,48, 51, 52, 56, 58, 59, 63, 73, 74, 77, 80, 83,84,85]. A greater number of studies showed an increased risk of exacerbations in females compared with males. In contrast, some studies failed to detect a relationship, suggesting that country-specific or cultural factors may play a role. A majority of the included studies evaluated more male patients than female patients; to further elucidate the relationship between sex and exacerbations, more studies in female patients are warranted. Over half of the studies that assessed the relationship between age and exacerbation risk found an association between increasing age and increasing risk of moderate-to-severe COPD exacerbations [14, 16, 18, 20,21,22,23,24, 26,27,28,29, 33, 40, 42, 44, 45, 47, 51, 52, 54, 56, 63, 73, 74, 77, 80, 83, 85].
Our findings also suggested that patients with low BMI have greater risk of moderate and/or severe exacerbations. The mechanism underlying this increased risk in underweight patients is poorly understood; however, loss of lean body mass in patients with COPD may be related to ongoing systemic inflammation that impacts skeletal muscle mass [101,102,103].
A limitation of this SLR, that may have resulted in some studies with valid results being missed, was the exclusion of non-English-language studies and the limitation by date; however, the search strategy was otherwise broad, resulting in the review of a large number of studies. The majority of studies captured in this SLR were from Europe, North America, and Asia. The findings may therefore be less generalizable to patients in other regions, such as Africa or South America. Given that one study reported an association between geographic location within different regions of the US and exacerbations [34], it is plausible that risk of exacerbations may be impacted by global location. As no formal meta-analysis was planned, the assessments are based on a qualitative synthesis of studies. A majority of the included studies looked at exposures of certain factors (e.g., history of exacerbations) at baseline; however, some of these factors change over time, calling into question whether a more sophisticated statistical analysis should have been conducted in some cases to consider time-varying covariates. Our results can only inform on associations, not causation, and there are likely bidirectional relationships between many factors and exacerbation risk (e.g., health status). Finally, while our review of the literature captured a large number of prognostic factors, other variables such as genetic factors, lung microbiome composition, and changes in therapy over time have not been widely studied to date, but might also influence exacerbation frequency [104]. Further research is needed to assess the contribution of these factors to exacerbation risk.
This SLR captured publications up to July 2019. However, further studies have since been published that further support the prognostic factors identified here. For example, recent studies have reported an increased risk of exacerbations in patients with a history of exacerbations [105], comorbidities [106], poorer lung function (GOLD stage) [105], higher symptomatic burden [107], female sex [105], and lower BMI [106, 108].
Conclusions
In summary, the literature assessing risk factors for moderate-to-severe COPD exacerbations shows that there are associations between several demographic and disease characteristics with COPD exacerbations, potentially allowing clinicians to identify patients most at risk of future exacerbations. Exacerbation history, comorbidities, and disease severity or bronchodilator reversibility were the factors most strongly associated with exacerbation risk, and should be considered in future research efforts to develop prognostic tools to estimate the likelihood of exacerbation occurrence. Importantly, many prognostic factors for exacerbations, such as symptom burden, QoL, and comorbidities, are modifiable with optimal pharmacologic and non-pharmacologic treatments or lifestyle modifications. Overall, the evidence suggests that, taken together, predicting and reducing exacerbation risk is an achievable goal in COPD.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CAT:
-
COPD Assessment Test
- COPD:
-
Chronic obstructive pulmonary disease
- FEV1 :
-
Forced expiratory volume in 1 s
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- ICS:
-
Inhaled corticosteroid
- mMRC:
-
Modified Medical Research Council
- OR:
-
Odds ratio
- QoL:
-
Quality of life
- SGRQ:
-
St. George’s Respiratory Questionnaire
- SLR:
-
Systematic literature review
References
World Health Organization. The top 10 causes of death. 2018. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 22 Jul 2020.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396:1204–22.
Hurst JR, Skolnik N, Hansen GJ, Anzueto A, Donaldson GC, Dransfield MT, Varghese P. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Intern Med. 2020;73:1–6.
Zhang Y, Morgan RL, Alonso-Coello P, Wiercioch W, Bała MM, Jaeschke RR, Styczeń K, Pardo-Hernandez H, Selva A, Ara Begum H, et al. A systematic review of how patients value COPD outcomes. Eur Respir J. 2018;52:1800222.
Global Initiative for Chronic Obstructive Lung Disease. 2022 GOLD Report. Global strategy for the diagnosis, management and prevention of COPD. 2022. https://goldcopd.org/2022-gold-reports-2/. Accessed 02 Feb 2022.
Müllerová H, Shukla A, Hawkins A, Quint J. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4: e006171.
Westerik JAM, Metting EI, van Boven JFM, Tiersma W, Kocks JWH, Schermer TR. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. 2017;18:31.
Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193:965–74.
Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerová H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Çolak Y, Afzal S, Marott JL, Nordestgaard BG, Vestbo J, Ingebrigtsen TS, Lange P. Prognosis of COPD depends on severity of exacerbation history: a population-based analysis. Respir Med. 2019;155:141–7.
Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198:464–71.
Pascoe S, Barnes N, Brusselle G, Compton C, Criner GJ, Dransfield MT, Halpin DMG, Han MK, Hartley B, Lange P, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7:745–56.
Yun JH, Lamb A, Chase R, Singh D, Parker MM, Saferali A, Vestbo J, Tal-Singer R, Castaldi PJ, Silverman EK, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10.
Yoon HY, Park SY, Lee CH, Byun MK, Na JO, Lee JS, Lee WY, Yoo KH, Jung KS, Lee JH. Prediction of first acute exacerbation using COPD subtypes identified by cluster analysis. Int J Chron Obstruct Pulmon Dis. 2019;14:1389–97.
Yohannes AM, Mulerova H, Lavoie K, Vestbo J, Rennard SI, Wouters E, Hanania NA. The association of depressive symptoms with rates of acute exacerbations in patients with COPD: results from a 3-year longitudinal follow-up of the ECLIPSE cohort. J Am Med Dir Assoc. 2017;18:955-959.e6.
Tsiligianni I, Metting E, van der Molen T, Chavannes N, Kocks J. Morning and night symptoms in primary care COPD patients: a cross-sectional and longitudinal study. An UNLOCK study from the IPCRG. NPJ Prim Care Respir Med. 2016;26:16040.
Jo YS, Yoon HI, Kim DK, Yoo CG, Lee CH. Comparison of COPD Assessment Test and Clinical COPD Questionnaire to predict the risk of exacerbation. Int J Chron Obstruct Pulmon Dis. 2018;13:101–7.
Marçôa R, Rodrigues DM, Dias M, Ladeira I, Vaz AP, Lima R, Guimarães M. Classification of Chronic Obstructive Pulmonary Disease (COPD) according to the new Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017: comparison with GOLD 2011. COPD. 2018;15:21–6.
Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, Cooper CB, Comellas A, Couper DJ, Curtis JL, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:619–26.
Yii ACA, Loh CH, Tiew PY, Xu H, Taha AAM, Koh J, Tan J, Lapperre TS, Anzueto A, Tee AKH. A clinical prediction model for hospitalized COPD exacerbations based on “treatable traits.” Int J Chron Obstruct Pulmon Dis. 2019;14:719–28.
McGarvey L, Lee AJ, Roberts J, Gruffydd-Jones K, McKnight E, Haughney J. Characterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med. 2015;109:228–37.
Margüello MS, Garrastazu R, Ruiz-Nuñez M, Helguera JM, Arenal S, Bonnardeux C, León C, Miravitlles M, García-Rivero JL. Independent effect of prior exacerbation frequency and disease severity on the risk of future exacerbations of COPD: a retrospective cohort study. NPJ Prim Care Respir Med. 2016;26:16046.
Engel B, Schindler C, Leuppi JD, Rutishauser J. Predictors of re-exacerbation after an index exacerbation of chronic obstructive pulmonary disease in the REDUCE randomised clinical trial. Swiss Med Wkly. 2017;147: w14439.
Benson VS, Müllerová H, Vestbo J, Wedzicha JA, Patel A, Hurst JR. Evaluation of COPD longitudinally to identify predictive surrogate endpoints (ECLIPSE) investigators. Associations between gastro-oesophageal reflux, its management and exacerbations of chronic obstructive pulmonary disease. Respir Med. 2015;109:1147–54.
Santibáñez M, Garrastazu R, Ruiz-Nuñez M, Helguera JM, Arenal S, Bonnardeux C, León C, García-Rivero JL. Predictors of hospitalized exacerbations and mortality in chronic obstructive pulmonary disease. PLoS ONE. 2016;11: e0158727.
Jo YS, Kim YH, Lee JY, Kim K, Jung KS, Yoo KH, Rhee CK. Impact of BMI on exacerbation and medical care expenses in subjects with mild to moderate airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2018;13:2261–9.
Alexopoulos EC, Malli F, Mitsiki E, Bania EG, Varounis C, Gourgoulianis KI. Frequency and risk factors of COPD exacerbations and hospitalizations: a nationwide study in Greece (Greek Obstructive Lung Disease Epidemiology and health ecoNomics: GOLDEN study). Int J Chron Obstruct Pulmon Dis. 2015;10:2665–74.
Liu D, Peng SH, Zhang J, Bai SH, Liu HX, Qu JM. Prediction of short term re-exacerbation in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1265–73.
Müllerová H, Maselli DJ, Locantore N, Vestbo J, Hurst JR, Wedzicha JA, Bakke P, Agusti A, Anzueto A. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147:999–1007.
de Miguel-Díez J, Hernández-Vázquez J, López-de-Andrés A, Álvaro-Meca A, Hernández-Barrera V, Jiménez-García R. Analysis of environmental risk factors for chronic obstructive pulmonary disease exacerbation: a case-crossover study (2004–2013). PLoS ONE. 2019;14: e0217143.
Krachunov II, Kyuchukov NH, Ivanova ZI, Yanev NA, Hristova PA, Borisova ED, Popova TP, Pavlov PS, Nikolova PT, Ivanov YY. Impact of air pollution and outdoor temperature on the rate of chronic obstructive pulmonary disease exacerbations. Folia Med (Plovdiv). 2017;59:423–9.
Baumeler L, Papakonstantinou E, Milenkovic B, Lacoma A, Louis R, Aerts JG, Welte T, Kostikas K, Blasi F, Boersma W, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology. 2016;21:883–90.
Annavarapu S, Goldfarb S, Gelb M, Moretz C, Renda A, Kaila S. Development and validation of a predictive model to identify patients at risk of severe COPD exacerbations using administrative claims data. Int J Chron Obstruct Pulmon Dis. 2018;13:2121–30.
Crisafulli E, Torres A, Huerta A, Méndez R, Guerrero M, Martinez R, Liapikou A, Soler N, Sethi S, Menéndez R. C-reactive protein at discharge, diabetes mellitus and ≥1 hospitalization during previous year predict early readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. COPD. 2015;12:311–20.
Bollmeier SG, Hartmann AP. Management of chronic obstructive pulmonary disease: a review focusing on exacerbations. Am J Health Syst Pharm. 2020;77:259–68.
Bafadhel M, Peterson S, De Blas MA, Calverley PM, Rennard SI, Richter K, Fagerås M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6:117–26.
Calverley PM, Anzueto AR, Dusser D, Mueller A, Metzdorf N, Wise RA. Treatment of exacerbations as a predictor of subsequent outcomes in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1297–308.
Calverley PM, Tetzlaff K, Dusser D, Wise RA, Mueller A, Metzdorf N, Anzueto A. Determinants of exacerbation risk in patients with COPD in the TIOSPIR study. Int J Chron Obstruct Pulmon Dis. 2017;12:3391–405.
Eklöf J, Sørensen R, Ingebrigtsen TS, Sivapalan P, Achir I, Boel JB, Bangsborg J, Ostergaard C, Dessau RB, Jensen US, et al. Pseudomonas aeruginosa and risk of death and exacerbations in patients with chronic obstructive pulmonary disease: an observational cohort study of 22 053 patients. Clin Microbiol Infect. 2020;26:227–34.
Estirado C, Ceccato A, Guerrero M, Huerta A, Cilloniz C, Vilaró O, Gabarrús A, Gea J, Crisafulli E, Soler N, Torres A. Microorganisms resistant to conventional antimicrobials in acute exacerbations of chronic obstructive pulmonary disease. Respir Res. 2018;19:119.
Fuhrman C, Moutengou E, Roche N, Delmas MC. Prognostic factors after hospitalization for COPD exacerbation. Rev Mal Respir. 2017;34:1–18.
Krachunov I, Kyuchukov N, Ivanova Z, Yanev NA, Hristova PA, Pavlov P, Glogovska P, Popova T, Ivanov YY. Stability of frequent exacerbator phenotype in patients with chronic obstructive pulmonary disease. Folia Med (Plovdiv). 2018;60:536–45.
Make BJ, Eriksson G, Calverley PM, Jenkins CR, Postma DS, Peterson S, Östlund O, Anzueto A. A score to predict short-term risk of COPD exacerbations (SCOPEX). Int J Chron Obstruct Pulmon Dis. 2015;10:201–9.
Montserrat-Capdevila J, Godoy P, Marsal JR, Barbé F. Predictive model of hospital admission for COPD exacerbation. Respir Care. 2015;60:1288–94.
Montserrat-Capdevila J, Godoy P, Marsal JR, Barbé F, Galván L. Risk factors for exacerbation in chronic obstructive pulmonary disease: a prospective study. Int J Tuberc Lung Dis. 2016;20:389–95.
Orea-Tejeda A, Navarrete-Peñaloza AG, Verdeja-Vendrell L, Jiménez-Cepeda A, González-Islas DG, Hernández-Zenteno R, Keirns-Davis C, Sánchez-Santillán R, Velazquez-Montero A, Puentes RG. Right heart failure as a risk factor for severe exacerbation in patients with chronic obstructive pulmonary disease: prospective cohort study. Clin Respir J. 2018;12:2635–41.
Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, Guasconi A, Montagna I, Vezzoli S, Petruzzelli S, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–84.
Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–80.
Pasquale MK, Xu Y, Baker CL, Zou KH, Teeter JG, Renda AM, Davis CC, Lee TC, Bobula J. COPD exacerbations associated with the modified Medical Research Council scale and COPD assessment test among Humana Medicare members. Int J Chron Obstruct Pulmon Dis. 2016;11:111–21.
Schuler M, Wittmann M, Faller H, Schultz K. Including changes in dyspnea after inpatient rehabilitation improves prediction models of exacerbations in COPD. Respir Med. 2018;141:87–93.
Singh D, Papi A, Corradi M, Pavlišová I, Montagna I, Francisco C, Cohuet G, Vezzoli S, Scuri M, Vestbo J. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388:963–73.
Søgaard M, Madsen M, Løkke A, Hilberg O, Sørensen HT, Thomsen RW. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int J Chron Obstruct Pulmon Dis. 2016;11:455–65.
Stanford RH, Nag A, Mapel DW, Lee TA, Rosiello R, Schatz M, Vekeman F, Gauthier-Loiselle M, Merrigan JFP, Duh MS. Claims-based risk model for first severe COPD exacerbation. Am J Manag Care. 2018;24:e45–53.
Stanford RH, Lau MS, Li Y, Stemkowski S. External validation of a COPD risk measure in a commercial and medicare population: the COPD treatment ratio. J Manag Care Spec Pharm. 2019;25:58–69.
Vestbo J, Papi A, Corradi M, Blazhko V, Montagna I, Francisco C, Cohuet G, Vezzoli S, Scuri M, Singh D. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919–29.
Wei X, Ma Z, Yu N, Ren J, Jin C, Mi J, Shi M, Tian L, Gao Y, Guo Y. Risk factors predict frequent hospitalization in patients with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:121–9.
Whalley D, Svedsater H, Doward L, Crawford R, Leather D, Lay-Flurrie J, Bosanquet N. Follow-up interviews from The Salford Lung Study (COPD) and analyses per treatment and exacerbations. NPJ Prim Care Respir Med. 2019;29:20.
Zeiger RS, Tran TN, Butler RK, Schatz M, Li Q, Khatry DB, Martin U, Kawatkar AA, Chen W. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract. 2018;6:944-954.e945.
Vogelmeier CF, Kostikas K, Fang J, Tian H, Jones B, Morgan CL, Fogel R, Gutzwiller FS, Cao H. Evaluation of exacerbations and blood eosinophils in UK and US COPD populations. Respir Res. 2019;20:178.
Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook R, Criner GJ, Franssen FME, Humbert M, Hurst JR, O’Donnell D, et al. An updated definition and severity classification of COPD exacerbations: the Rome proposal. Am J Respir Crit Care Med. 2021;204:1251–8.
Adir Y, Hakrush O, Shteinberg M, Schneer S, Agusti A. Circulating eosinophil levels do not predict severe exacerbations in COPD: a retrospective study. ERJ Open Research. 2018;4:00022–2018.
Bartels W, Adamson S, Leung L, Sin DD, van Eeden SF. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease: factors predicting readmission. Int J Chron Obstruct Pulmon Dis. 2018;13:1647–54.
Kim V, Zhao H, Regan E, Han MK, Make BJ, Crapo JD, Jones PW, Curtis JL, Silverman EK, Criner GJ, COPDGene Investigators. The St. George’s Respiratory Questionnaire definition of chronic bronchitis may be a better predictor of COPD exacerbations compared with the classic definition. Chest. 2019;156:685–95.
Abston E, Comellas A, Reed RM, Kim V, Wise RA, Brower R, Fortis S, Beichel R, Bhatt S, Zabner J, et al. Higher BMI is associated with higher expiratory airflow normalised for lung volume (FEF25-75/FVC) in COPD. BMJ Open Respir Res. 2017;4: e000231.
Emura I, Usuda H, Satou K. Appearance of large scavenger receptor A-positive cells in peripheral blood: a potential risk factor for severe exacerbation of chronic obstructive pulmonary disease. Pathol Int. 2019;69:187–92.
Erol S, Sen E, Gizem Kilic Y, Yousif A, Akkoca Yildiz O, Acican T, Saryal S. Does the 2017 revision improve the ability of GOLD to predict risk of future moderate and severe exacerbation? Clin Respir J. 2018;12:2354–60.
Han MZ, Hsiue TR, Tsai SH, Huang TH, Liao XM, Chen CZ. Validation of the GOLD 2017 and new 16 subgroups (1A–4D) classifications in predicting exacerbation and mortality in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3425–33.
Huang TH, Hsiue TR, Lin SH, Liao XM, Su PL, Chen CZ. Comparison of different staging methods for COPD in predicting outcomes. Eur Resp J. 2018;51:1700577.
Jung YH, Lee DY, Kim DW, Park SS, Heo EY, Chung HS, Kim DK. Clinical significance of laryngopharyngeal reflux in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1343–51.
Kim J, Kim WJ, Lee CH, Lee SH, Lee MG, Shin KC, Yoo KH, Lee JH, Lim SY, Na JO, et al. Which bronchodilator reversibility criteria can predict severe acute exacerbation in chronic obstructive pulmonary disease patients? Respir Res. 2017;18:107.
Kobayashi S, Hanagama M, Ishida M, Sato H, Ono M, Yamanda S, Yamada M, Aizawa H, Yanai M. Clinical characteristics and outcomes in Japanese patients with COPD according to the 2017 GOLD classification: the Ishinomaki COPD Network Registry. Int J Chron Obstruct Pulmon Dis. 2018;13:3947–55.
Lee SH, Lee JH, Yoon HI, Park HY, Kim TH, Yoo KH, Oh YM, Jung KS, Lee SD, Lee SW. Change in inhaled corticosteroid treatment and COPD exacerbations: an analysis of real-world data from the KOLD/KOCOSS cohorts. Respir Res. 2019;20:62.
Pavlovic R, Stefanovic S, Lazic Z, Jankovic S. Factors associated with the rate of COPD exacerbations that require hospitalization. Turk J Med Sci. 2017;47:134–41.
Song JH, Lee CH, Um SJ, Park YB, Yoo KH, Jung KS, Lee SD, Oh YM, Lee JH, Kim EK, Kim DK. Clinical impacts of the classification by 2017 GOLD guideline comparing previous ones on outcomes of COPD in real-world cohorts. Int J Chron Obstruct Pulmon Dis. 2018;13:3473–84.
Sundh J, Johansson G, Larsson K, Lindén A, Löfdahl CG, Sandström T, Janson C. The phenotype of concurrent chronic bronchitis and frequent exacerbations in patients with severe COPD attending Swedish secondary care units. Int J Chron Obstruct Pulmon Dis. 2015;10:2327–34.
Urwyler P, Hussein NA, Bridevaux PO, Chhajed PN, Geiser T, Grendelmeier P, Zellweger LJ, Kohler M, Maier S, Miedinger D, et al. Predictive factors for exacerbation and reexacerbation in chronic obstructive pulmonary disease: an extension of the Cox model to analyze data from the Swiss COPD cohort. Multidiscip Respir Med. 2019;14:7.
Wallace AE, Kaila S, Bayer V, Shaikh A, Shinde MU, Willey VJ, Napier MB, Singer JR. Health care resource utilization and exacerbation rates in patients with COPD stratified by disease severity in a commercially insured population. J Manag Care Spec Pharm. 2019;25:205–17.
Halpin DMG, Decramer M, Celli BR, Mueller A, Metzdorf N, Tashkin DP. Effect of a single exacerbation on decline in lung function in COPD. Respir Med. 2017;128:85–91.
Bade BC, DeRycke EC, Ramsey C, Skanderson M, Crothers K, Haskell S, Bean-Mayberry B, Brandt C, Bastian LA, Akgün KM. Sex differences in veterans admitted to the hospital for chronic obstructive pulmonary disease exacerbation. Ann Am Thorac Soc. 2019;16:707–14.
Iyer AS, Bhatt SP, Dransfield M, Kinney G, Holm K, Wamboldt FS, Hanania N, Martinez C, Regan E, Foreman MG, et al. Psychological distress prospectively predicts severe exacerbations in smokers with and without airflow limitation—a longitudinal follow-up study of the COPDGene cohort [abstract]. Am J Respir Crit Care Med. 2017. https://doi.org/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A4709.
Diamond M, Zhao H, Armstrong HF, Morrison M, Bailey KL, Carretta EE, Criner GJ, Han MK, Bleeker E, Cooper CB, et al. Anxiety and depression, either alone or in combination, are associated with respiratory exacerbations in smokers with and without COPD [abstract]. Am J Respir Crit Care Med. 2017;195:1615–31.
Lau CS, Siracuse BL, Chamberlain RS. Readmission After COPD Exacerbation Scale: determining 30-day readmission risk for COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:1891–902.
Pikoula M, Quint JK, Nissen F, Hemingway H, Smeeth L, Denaxas S. Identifying clinically important COPD sub-types using data-driven approaches in primary care population based electronic health records. BMC Med Inform Decis Mak. 2019;19:86.
Wei YF, Tsai YH, Wang CC, Kuo PH. Impact of overweight and obesity on acute exacerbations of COPD—subgroup analysis of the Taiwan Obstructive Lung Disease cohort. Int J Chron Obstruct Pulmon Dis. 2017;12:2723–9.
Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Resp J. 2009;33:1165–85.
Polverino E, Dimakou K, Hurst J, Martinez-Garcia MA, Miravitlles M, Paggiaro P, Shteinberg M, Aliberti S, Chalmers JD. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J. 2018;52:1800328.
Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Platt RW, Wang C, Bourbeau J. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care Med. 2008;178:913–20.
Chapman KR, Hurst JR, Frent SM, Larbig M, Fogel R, Guerin T, Banerji D, Patalano F, Goyal P, Pfister P, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198:329–39.
Couillard S, Larivée P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151:366–73.
Ferguson GT, Rabe KF, Martinez FJ, Fabbri LM, Wang C, Ichinose M, Bourne E, Ballal S, Darken P, DeAngelis K, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6:747–58.
Ko FWS, Chan KP, Ngai J, Ng SS, Yip WH, Ip A, Chan TO, Hui DSC. Blood eosinophil count as a predictor of hospital length of stay in COPD exacerbations. Respirology. 2019;25:259–66.
MacDonald MI, Osadnik CR, Bulfin L, Hamza K, Leong P, Wong A, King PT, Bardin PG. Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest. 2019;156:92–100.
Müllerová H, Hahn B, Simard EP, Mu G, Hatipoğlu U. Exacerbations and health care resource use among patients with COPD in relation to blood eosinophil counts. Int J Chron Obstruct Pulmon Dis. 2019;14:683–92.
Bafadhel M, Greening NJ, Harvey-Dunstan TC, Williams JEA, Morgan MD, Brightling CE, Hussain SF, Pavord ID, Singh SJ, Steiner MC. Blood eosinophils and outcomes in severe hospitalised exacerbations of COPD. Chest. 2016;150:320–8.
Roche N, Chapman KR, Vogelmeier CF, Herth FJF, Thach C, Fogel R, Olsson P, Patalano F, Banerji D, Wedzicha JA. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med. 2017;195:1189–97.
Vestbo J, Vogelmeier CF, Small M, Siddall J, Fogel R, Kostikas K. Inhaled corticosteroid use by exacerbations and eosinophils: a real-world COPD population. Int J Chron Obstruct Pulmon Dis. 2019;14:853–61.
Watz H, Tetzlaff K, Wouters EFM, Kirsten A, Magnussen H, Rodriguez-Roisin R, Vogelmeier C, Fabbri LM, Chanez P, Dahl R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4:390–8.
Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, Barnes NC. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax. 2016;71:118–25.
Singh D. Predicting corticosteroid response in chronic obstructive pulmonary disease. Blood eosinophils gain momentum. Am J Respir Crit Care Med. 2017;196:1098–100.
Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sørensen TIA, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83.
Agustí AGN, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347–60.
Agustí AGN, Sauleda J, Miralles C, Gomez C, Togores B, Sala E, Batle S, Busquets X. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:485–9.
Labaki WW, Martinez FJ. Time to understand the infrequency of the frequent exacerbator phenotype in COPD. Chest. 2018;153:1087–8.
Hartley BF, Barnes NC, Lettis S, Compton CH, Papi A, Jones P. Risk factors for exacerbations and pneumonia in patients with chronic obstructive pulmonary disease: a pooled analysis. Respir Res. 2020;21:5.
Kim Y, Kim YJ, Kang YM, Cho WK. Exploring the impact of number and type of comorbidities on the risk of severe COPD exacerbations in Korean Population: a Nationwide Cohort Study. BMC Pulm Med. 2021;21:151.
Mackay AJ, Kostikas K, Roche N, Frent SM, Olsson P, Pfister P, Gupta P, Patalano F, Banerji D, Wedzicha JA. Impact of baseline symptoms and health status on COPD exacerbations in the FLAME study. Respir Res. 2020;21:93.
Smulders L, van der Aalst A, Neuhaus EDET, Polman S, Franssen FME, van Vliet M, de Kruif MD. Decreased risk of COPD exacerbations in obese patients. COPD. 2020;17:485–91.
Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, Citrome L, Gurr JA, Mooney LA, Moore BJ, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163:461–4.
Putcha N, Barr RG, Han M, Woodruff PG, Bleecker ER, Kanner RE, Martinez FJ, Tashkin DP, Rennard SI, Breysse P, et al. Understanding the impact of passive smoke exposure on outcomes in COPD [abstract]. Am J Respir Crit Care Med. 2015;191:411–20.
Wu Z, Yang D, Ge Z, Yan M, Wu N, Liu Y. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: a retrospective real world research. J Thorac Dis. 2018;10:5086–99.
Acknowledgements
Medical writing support, under the direction of the authors, was provided by Julia King, PhD, and Sarah Piggott, MChem, CMC Connect, McCann Health Medical Communications, funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines [109].
Funding
This study was supported by AstraZeneca.
Author information
Authors and Affiliations
Contributions
The authors have made the following declaration about their contributions. JRH and MKH made substantial contributions to the interpretation of data; BS, SS, GK, and MKS made substantial contributions to the acquisition, analysis, and interpretation of data; EdN and UH made substantial contributions to the conception and design of the work and the interpretation of data. All authors contributed to drafting or critically revising the article, have approved the submitted version, and agree to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JRH reports consulting fees from AstraZeneca; speaker fees from AstraZeneca, Chiesi, Pfizer, and Takeda; and travel support from GlaxoSmithKline and AstraZeneca. MKH reports assistance with conduction of this research and publication from AstraZeneca; personal fees from Aerogen, Altesa Biopharma, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, DevPro, GlaxoSmithKline, Integrity, Medscape, Merck, Mylan, NACE, Novartis, Polarean, Pulmonx, Regeneron, Sanofi, Teva, Verona, United Therapeutics, and UpToDate; either in kind research support or funds paid to the institution from the American Lung Association, AstraZeneca, Biodesix, Boehringer Ingelheim, the COPD Foundation, Gala Therapeutics, the NIH, Novartis, Nuvaira, Sanofi, and Sunovion; participation in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution; and stock options from Altesa Biopharma and Meissa Vaccines. BS, GK, and MKS are former employees of Parexel International. SS is an employee of Parexel International, which was funded by AstraZeneca to conduct this analysis. EdN is a former employee of AstraZeneca and previously held stock and/or stock options in the company. UH is an employee of AstraZeneca and holds stock and/or stock options in the company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1: Table S1.
Search strategies. Table S2. List of included studies with linked publications. Table S3. Study characteristics across the 76 included studies. Table S4. Clinical characteristics of the patients assessed across the included studies.
Additional file 2: Fig. S1.
Sex (male vs female) as a risk factor for moderate-to-severe exacerbations. Fig. S2. Sex (male vs female) as a risk factor for severe exacerbations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hurst, J.R., Han, M.K., Singh, B. et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res 23, 213 (2022). https://doi.org/10.1186/s12931-022-02123-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02123-5