- Research
- Open access
- Published:
Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease
Respiratory Research volume 22, Article number: 271 (2021)
Abstract
Background
In patients with chronic obstructive pulmonary disease (COPD), the maximum level of diaphragm excursion (DEmax) is correlated with dynamic lung hyperinflation and exercise tolerance. This study aimed to elucidate the utility of DEmax to predict the improvement in exercise tolerance after pulmonary rehabilitation (PR) in patients with COPD.
Methods
This was a prospective cohort study. Of the 62 patients with stable COPD who participated in the outpatient PR programme from April 2018 to February 2021, 50 completed the programme. Six-minute walk distance (6MWD) was performed to evaluate exercise tolerance, and ultrasonography was performed to measure DEmax. Responders to PR in exercise capacity were defined as patients who demonstrated an increase of > 30 m in 6MWD. The receiver operating characteristic (ROC) curve was used to determine the cut-off point of DEmax to predict responses to PR.
Results
Baseline levels of forced expiratory volume in 1 s, 6MWD, maximum inspiratory pressure, DEmax and quadriceps muscle strength were significantly higher, and peak dyspnoea of modified Borg (mBorg) scale score was lower in responders (n = 30) than in non-responders (n = 20) to PR (p < 0.01). In multivariate analysis, DEmax was significantly correlated with an increase of > 30 m in 6MWD. The area under the ROC curve of DEmax to predict responders was 0.915, with a sensitivity and specificity of 83% and 95%, respectively, at a cut-off value of 44.9 mm of DEmax.
Conclusion
DEmax could adequately predict the improvement in exercise tolerance after PR in patients with COPD.
Background
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterised by minimally reversible airflow limitation [1]. The main feature of COPD is the inability of patients to cope with their activities of daily life due to shortness of breath. Although the pathophysiological mechanisms involved in the development of dyspnoea and poor exercise tolerance in patients with COPD are complex, dynamic lung hyperinflation (DLH) plays a central role [2] by increasing ventilatory workload and decreasing the pressure-generating capacity of the inspiratory muscles.
Pulmonary rehabilitation (PR) is a non-pharmacological intervention and has been reported to improve dyspnoea, exercise capacity and quality of life of patients with COPD [3]. Owing to a body of evidence, PR is now established as the standard of care for patients with COPD [4]. However, not all patients with COPD benefit from PR to the same extent. Therefore, identifying patients who are likely to achieve maximum benefit from the PR programme is crucial. So far, several studies have shown that severe airflow limitation or poor exercise tolerance at baseline may predict a better response to PR [5, 6], but another study has reported inconsistent findings [7]. Furthermore, one study reported that patients with severe dyspnoea did not respond well to PR and patients with milder dyspnoea responded well [8].
Considering the role of DLH in the development of dyspnoea and poor exercise tolerance in patients with COPD, objective measures that reflect the degree of DLH may help in identifying good responders to PR. Previously, we reported that there was an association between increased dyspnoea due to DLH on exercise and decreased exercise capacity in patients with COPD and reduced mobility of the diaphragm, which was assessed by the maximum level of diaphragm excursion (DEmax) using ultrasonography [9]. Other research groups reported the utility of ultrasonographic assessment of diaphragmatic mobility in COPD in understanding its association with 6-min walk distance (6MWD), dyspnoea [10] and increased mortality [11].
However, there have been no reports on the association between diaphragmatic mobility and the effect of PR to improve exercise tolerance. The primary aim of this study is to clarify the role of DEmax to predict the improvement in exercise tolerance after PR in patients with COPD.
Materials and methods
Study design and subjects
This was a single-centre, observational, prospective cohort study. The study included 62 patients with clinically stable COPD who visited the Department of Respiratory Medicine and Allergology, Kindai University Hospital, between April 2018 and February 2021. The exclusion criteria included unstable medical conditions that could cause or contribute to breathlessness, such as metabolic, cardiovascular or other respiratory diseases, or any other disorders that could interfere with exercise testing, such as neuromuscular diseases or musculoskeletal problems. This study was approved by the Ethics Committee of Kindai University School of Medicine. Written informed consent was obtained from all participants.
Measurements
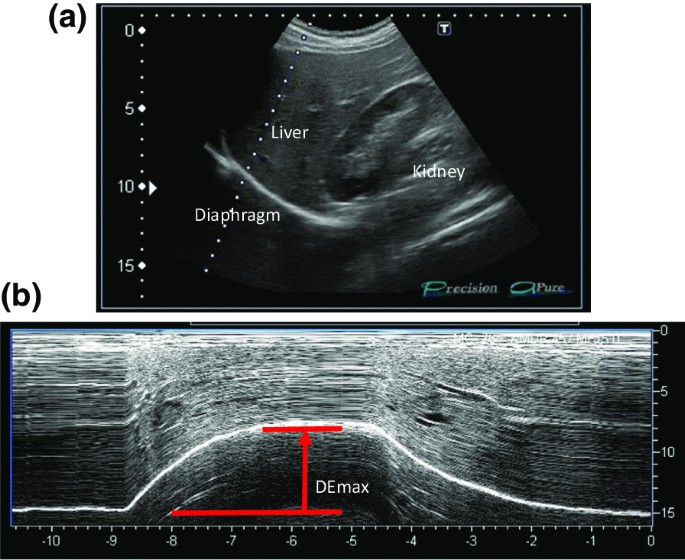
All participants underwent ultrasonography (Xario 200, Toshiba, Tokyo, Japan) for the assessment of their DEmax. Using the liver as an acoustic window (Fig. 1A), a convex 3.5 MHz probe was used to measure the excursions of the right hemidiaphragm according to the techniques mentioned in previous studies [9, 12, 13]. The M-mode cursor was rotated and placed on the axis of diaphragmatic displacement on the stored image, and displacement measurements were performed. Measurements were performed during each of the three deep breaths, and DEmax was measured (Fig. 1B). The maximum value obtained for the three deep breaths was used. 6MWD was performed to evaluate walking capacity according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) statement [14,15,16]. All participants performed the 6MWD test before and after the PR programme, and the magnitude of their perceived breathlessness and their leg fatigue was rated using a 1–10-point Borg scale. Responders to PR in exercise capacity were defined as those who demonstrated more than 30 m increase in 6MWD after the PR programme, which was the definition of minimal clinically important difference (MCID) for 6MWD [17].
Representative image of the right diaphragm. The probe was positioned below the right costal margin between the midclavicular and anterior axillary lines. A Two-dimensional ultrasonographic image of the right hemidiaphragm (B-mode). Diaphragmatic movements were recorded in M-mode during deep breathing (DEmax) (B)
Spirometry (CHESTAC-800, Chest, Tokyo, Japan) was performed following the 2005 ATS/ERS recommendations [18] for measuring forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1) and inspiratory capacity. Respiratory muscle strength was assessed by measuring the maximum inspiratory pressure (PImax) generated against an occluded airway at residual volume [19] (SP-370, Fukuda Denshi, Tokyo, Japan). A hand-held dynamometer (μTasF-1, Anima Corp., Tokyo) was used to measure quadriceps muscle strength (QMS). The impact of COPD on health status was assessed using the COPD assessment test (CAT), a patient-completed questionnaire on eight items, namely, cough, phlegm, chest tightness, breathlessness, limited activities, confidence leaving home, sleeplessness and energy. The scores for each of the items range from 0 to 5 points, resulting in a CAT total score ranging from 0 to 40 points [20], and MCID of CAT is 2 points [21]. In all patients with COPD, emphysema was evaluated by computed tomography of the chest. A SYNAPSE VINCENT volume analyser (FUJIFILM Medical, Tokyo, Japan) was used to measure the low attenuation area (%LAA).
Rehabilitation programme
The outpatient PR programme was conducted twice a week for 12 weeks (24 sessions), including aerobic exercise training (ergometer and walking exercise) at 60–70% of peak workload for 20–40 min and upper- and lower-limb muscle strength training for 10–20 min.
Sample size
The sample size was estimated using R software. The analysis based on 6MWD data from the PR programme revealed that 40 subjects were required if the expected area under the curve (AUC) below the receiver operating characteristic (ROC) curve was 0.80, the power was 90%, and the significance level was 0.01. Furthermore, we anticipated a dropout from the PR programme. Thus, we set the sample size to 50 participants.
Statistical analysis
Responders and non-responders were compared using t-test, the Wilcoxon rank-sum test or χ2 test, as appropriate. The paired t-test or the Wilcoxon signed-rank test was used to evaluate the changes in the parameters before and after the PR programme. The Pearson correlation coefficient was used to analyse the relationship between changes in 6MWD and independent variables because changes in 6MWD were normally distributed. Additionally, multivariate logistic regression models were used to assess the ability of variables to predict a response to PR. The ROC curve method was used to assess the ability of DEmax to predict a response to PR. All statistical analyses were performed using the JMP software programme (JMP®, Version 14; SAS Institute Inc., Cary, NC, USA).
Result
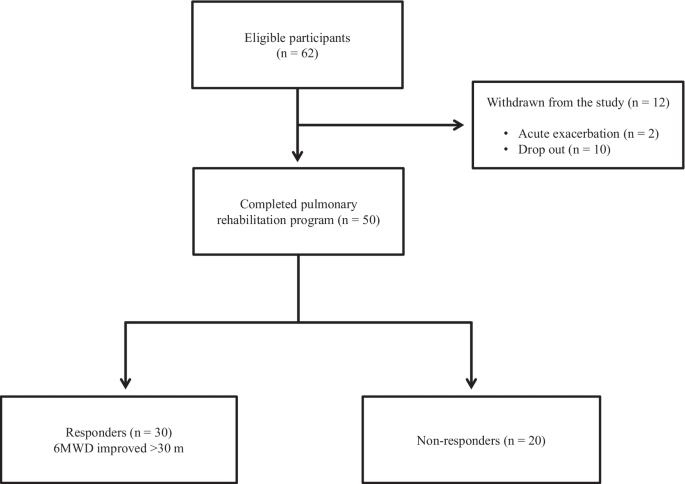
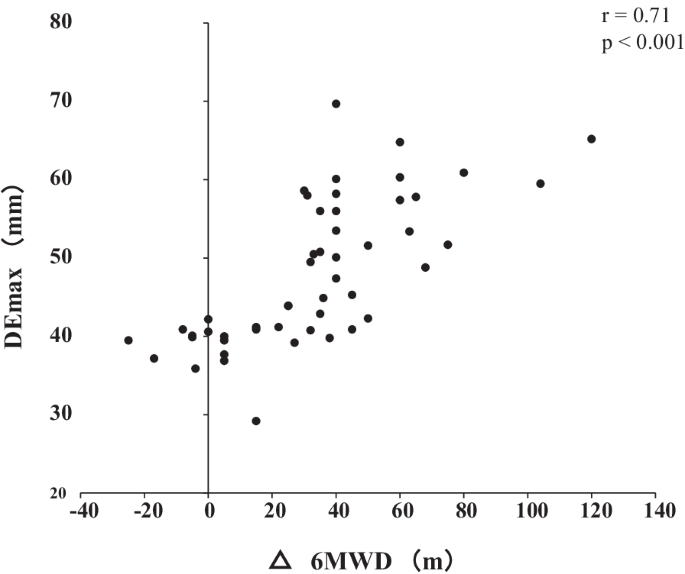
Out of the 62 patients included in the study, 50 completed the PR programme (Fig. 2). Two patients dropped out because of severe exacerbation of COPD, and 10 patients discontinued the PR owing to the coronavirus pandemic. Table 1 presents the baseline characteristics of the participants. After the PR programme, scores for CAT, 6MWD, peak dyspnoea and leg fatigue of the modified Borg (mBorg) scale, and QMS improved significantly (Table 2). Thirty patients showed an increase of > 30 m in 6MWD after PR (responders: 60%), and 20 patients (40%) were defined as non-responders. Baseline levels of %FEV1, 6MWD, PImax, DEmax and QMS were significantly higher and those of CAT score and peak dyspnoea of mBorg scale were significantly lower in responders than in non-responders (Table 1). Changes in 6MWD were significantly correlated with baseline levels of CAT, %FEV1, peak dyspnoea of mBorg scale, PImax, DEmax (Fig. 3) and QMS and marginally correlated with baseline levels of 6MWD (Table 3).
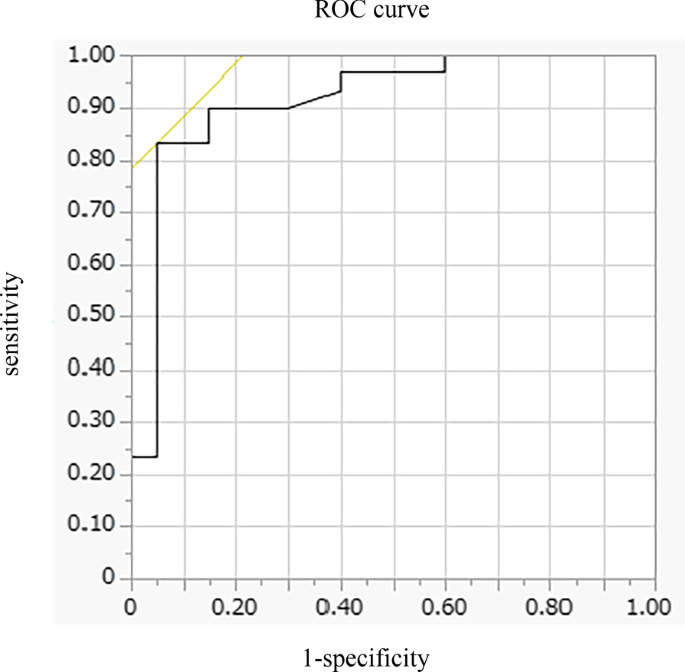
In multivariate analysis, DEmax alone significantly contributed to the prediction of responders (Table 4, Model 1). When using PImax instead of DEmax because PImax and DEmax showed a strong association (r = 0.73), both PImax and %FEV1 contributed to the prediction (Table 4, Model 2). The area under the ROC curve of DEmax to predict the responders was 0.915, with a sensitivity of 83% and a specificity of 95% at a cut-off value of 44.9 mm of DEmax (Fig. 4). The significance of DEmax in the predictability of responders remained even when the analysis was confined to severe patients (%FEV1 < 50%, n = 23; AUC = 0.88, sensitivity = 70% and specificity = 100% at a cut-off value of 44.9 mm).
Receiver operating characteristic (ROC) curve for baseline DEmax in relation to the response to pulmonary rehabilitation. ROC curve estimates the ability of DEmax to predict a clinically important improvement in 6MWD (> 30 m) after pulmonary rehabilitation (AUC = 0.915, sensitivity = 83% and specificity = 95% at a cut-off point of 44.9 mm of DEmax). AUC area under the curve, 6MWD 6-min walk distance, DEmax maximum diaphragmatic excursion
Discussion
This is the first study to demonstrate the utility of DEmax to predict the responsiveness of patients with COPD to 12-week PR. In this study, multivariate analysis revealed that greater baseline DEmax was the only factor that predicted the responsiveness to PR, independent of baseline %FEV1. Additionally, the model using DEmax had better prediction performance than that using PImax. The AUC of DEmax to predict the 30 m or more improvement in 6MWD after the PR was 0.915, with a sensitivity of 83% and a specificity of 95% at 44.9 mm.
PR is beneficial to patients with chronic respiratory disease, including COPD [3], and generally improves exercise performance, health-related quality of life and dyspnoea [22], which was confirmed in this study. Ideally, PR was proven to be effective in all patients, but the response to PR varies considerably between individual patients [8, 23,24,25]. Indeed, in this study, the improvement in 6MWD was less than that in MCID in 40% of the patients regardless of the degree of severity of COPD. Therefore, identifying predictors of a response is crucial in ensuring better PR efficacy and personalisation of PR programmes for patients with COPD.
In this study, the baseline values of %FEV1, PImax, DEmax, QMS and 6MWD were positively associated with Δ6MWD in univariate analysis, suggesting that a better baseline condition was associated with a higher proportion of patients who achieved MCID after PR. These findings are consistent with those of previous studies that showed that patients with higher levels of %FEV1 or FEV1/VC achieved greater improvement in 6MWD after PR [7, 26, 27] and a study in which patients with milder mMRC scores could achieve MCID of 6MWD after PR [8], but not for those with worst mMRC score, although others studies showed contradictory results [5, 6, 28,29,30] or found no significant baseline characteristics to predict a response to PR [31]. The discrepancy between the findings cannot be fully explained, but it might be due to the differences in the studied population and strength or length of PR. In this study, the mean %FEV1 of the participants was 56.0%, which was relatively higher than that of other studies (mean %FEV1 of 40–50% in most studies) [5, 6, 28], despite similar inclusion criteria throughout the studies, i.e., not limited to severe COPD in most studies. Thus, no ceiling effect with a PR programme that included high-intensity load exercise training for 20–40 min was observed in our population.
In this study, an important finding is that greater DEmax at baseline was the only factor that predicted the responders in 6MWD after PR. In addition, the model using DEmax had better prediction performance than that using PImax. The high predictability of DEmax may be because of its strong association with DLH and dyspnoea during exercise, as reported previously [9]. DLH is involved in the development of dyspnoea, and both are important factors to determine the improvement in 6MWD in patients with COPD. Therefore, DEmax that reflects the degree of DLH and dyspnoea during exercise was superior to other physiological indices to predict responders.
Furthermore, the virtuous cycle observed in our PR programme that included high-intensity load exercise training might be a result of the improvement in ventilation pattern. Improving the ventilation pattern would be easier with greater DEmax, as shown in studies of mechanically ventilated patients [32], which may have reduced dyspnoea during exercise after 12 weeks of PR and improved exercise tolerance. Exercise therapy is a central component of PR, which significantly reduces blood lactate levels during exercise, reduces minute ventilation and improves exercise tolerance [33]. The high-intensity load exercise training, which is performed at 60–80% of the maximum oxygen uptake, has a higher physiological effect than low exercise load. Patients with greater DEmax may be able to perform higher load training, which resulted in effective PR.
Diaphragm ultrasonography has been widely and successfully used to identify diaphragmatic dysfunction by showing its association with 6MWD, dyspnoea [10], extubation failure in mechanically ventilated patients [32], and increased mortality [11]. Recently, Lewinska and Shahnazzaryan proposed its use in pulmonary physiotherapy of patients with COPD [34]. In most previous studies, diaphragm ultrasonography was used to assess DEmax, i.e., the measurement of the excursion of the right hemidiaphragm, as used in this study, and diaphragm thickness that assessed the length and thickness of the zone of apposition of the diaphragm against the rib cage [35, 36]. However, it is difficult to measure diaphragm thickness in patients with severe COPD because the length of the zone of apposition is shorter in patients with COPD than that in control subjects [37], whereas it is easy to measure DEmax, which shows high intra- and inter-observer reliability [38]. Bhatt et al. showed that improvement in 6MWD was associated with that in DEmax during forced expiration when the effectiveness of pursed lips breathing was assessed in the PR of patients with COPD [39]. Corbellini et al. demonstrated greater improvement in DEmax during inspiration after PR, which was associated with an increase in the inspiratory capacity [40]. The normal and cut-off values of DEmax during normal respiration, forced respiration, and voluntary sniffing have been described for each gender [38]. Thus, DEmax would be a useful and reliable measure for incorporation into the PR assessment. Furthermore, in clinical settings, this objective measure of DEmax has additional advantages as it requires minimum effort in patients and can be applied to the PR programme at home if portable ultrasonography is used. However, the assessment of DEmax has a limitation. The procedures pertaining to positioning of patients, breathing patterns, and the selected hemidiaphragm are not standardised at present, which may hamper the routine use of DEmax at this moment. Standardisation of these parameters would further facilitate the use of DEmax in clinical settings and for research purpose.
There are some limitations to this study. This was a single-centre study involving a relatively small number of participants, and their baseline condition might have been relatively preserved. Nonetheless, 46% of the participants showed FEV1 < 50%, and the utility of DEmax was also observed in these patients with severe airflow limitation. Furthermore, in this study, few patients discontinued the PR programme, except for patients who discontinued during the coronavirus pandemic, which indicates that there was no severe mismatch between the PR programme and the patients’ ability to successfully complete this programme. As another limitation, we did not evaluate any malnutrition factors, which could be an important determinant of diaphragmatic mobility. Nonetheless, DEmax was a stronger predictor of the effectiveness of PR than other parameters, including QMS or lung function using multivariate analysis. Further studies with a large number of patients are required, and the utility of DEmax should be examined in patients with the most severe form of COPD with a low-intensity load exercise programme.
Conclusion
In conclusion, DEmax, which is a reliable and easy to perform measurement, could adequately predict the improvement in exercise tolerance after PR in patients with COPD. Assessment of DEmax could aid in making medical decisions associated with therapeutic strategies.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- DLH:
-
Dynamic lung hyperinflation
- PR:
-
Pulmonary rehabilitation
- 6MWD:
-
6-Min walk distance
- MCID:
-
Minimal clinically important difference
- FVC:
-
Forced vital capacity
- FEV1:
-
Forced expiratory volume in 1 s
- PImax:
-
Maximum inspiratory pressure
- QMS:
-
Quadriceps muscle strength
- CAT:
-
COPD assessment test
- %LAA:
-
Low attenuation area
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
- mBorg:
-
Modified Borg
References
Global initiative for chronic obstructive lung disease (gold). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2020 report. . https://goldcopd.org/gold-reports/ last accessed: 20 Jan 2020.
Gagnon P, Guenette JA, Langer D, Laviolette L, Mainguy V, Maltais F, Ribeiro F, Saey D. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J COPD. 2014;9:187–201.
Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-64.
Dong J, Li Z, Luo L, Xie H. Efficacy of pulmonary rehabilitation in improving the quality of life for patients with chronic obstructive pulmonary disease: evidence based on nineteen randomized controlled trials. Int J Surg. 2020;73:78–86.
Boutou AK, Tanner RJ, Lord VM, Hogg L, Nolan J, Jefford H, Corner EJ, Falzon C, Lee C, Garrod R, et al. An evaluation of factors associated with completion and benefit from pulmonary rehabilitation in COPD. BMJ Open Respir Res. 2014;1:e000051.
Costi S, Crisafulli E, Trianni L, Beghe B, Faverzani S, Scopelliti G, Chetta A, Clini E. Baseline exercise tolerance and perceived dyspnea to identify the ideal candidate to pulmonary rehabilitation: a risk chart in COPD patients. Int J Chron Obstruct Pulmon Dis. 2019;14:3017–23.
van Ranst D, Otten H, Meijer JW, van’t Hul AJ. Outcome of pulmonary rehabilitation in COPD patients with severely impaired health status. Int J Chron Obstruct Pulmon Dis. 2011;6:647–57.
Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J. 2006;27:788–94.
Shiraishi M, Higashimoto Y, Sugiya R, Mizusawa H, Takeda Y, Fujita S, Nishiyama O, Kudo S, Kimura T, Chiba Y, et al. Diaphragmatic excursion correlates with exercise capacity and dynamic hyperinflation in COPD patients. ERJ Open Res 2020, 6.
Paulin E, Yamaguti WPS, Chammas MC, Shibao S, Stelmach R, Cukier A, Carvalho CRF. Influence of diaphragmatic mobility on exercise tolerance and dyspnea in patients with COPD. Respir Med. 2007;101:2113–8.
Yamaguti WPdS, Paulin E, Salge JM, Chammas MC, Cukier A, de Carvalho CRF. Diaphragmatic dysfunction and mortality in patients with COPD. J Bras Pneumol. 2009;35:1174–81.
Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135:391–400.
Testa A, Soldati G, Giannuzzi R, Berardi S, Portale G, Gentiloni Silveri N. Ultrasound M-Mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol. 2011;37:44–52.
Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7.
Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, McCormack MC, Carlin BW, Sciurba FC, Pitta F, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–46.
Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, Lee AL, Camillo CA, Troosters T, Spruit MA, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–78.
Polkey MI, Spruit MA, Edwards LD, Watkins ML, Pinto-Plata V, Vestbo J, Calverley PMA, Tal-Singer R, Agustí A, Bakke PS, et al. Six-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:382–6.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Lisboa C, Munoz V, Beroiza T, Leiva A, Cruz E. Inspiratory muscle training in chronic airflow limitation: comparison of two different training loads with a threshold device. Eur Respir J. 1994;7:1266–74.
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54.
Kon SSC, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, Haselden BM, Polkey MI, Man WDC. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2:195–203.
Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006:CD003793.
Spruit MA, Gosselink R, Troosters T, Kasran A, Van Vliet M, Decramer M. Low-grade systemic inflammation and the response to exercise training in patients with advanced COPD. Chest. 2005;128:3183–90.
de Torres JP, Pinto-Plata V, Ingenito E, Bagley P, Gray A, Berger R, Celli B. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121:1092–8.
Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from nonresponders. J Cardiopulm Rehabil. 2001;21:10–7.
Vagaggini B, Costa F, Antonelli S, De Simone C, De Cusatis G, Martino F, Santerini S, Paggiaro P. Clinical predictors of the efficacy of a pulmonary rehabilitation programme in patients with COPD. Respir Med. 2009;103:1224–30.
Scott AS, Baltzan MA, Fox J, Wolkove N. Success in pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Can Respir J. 2010;17:219–23.
Crisafulli E, Gorgone P, Vagaggini B, Pagani M, Rossi G, Costa F, Guarriello V, Paggiaro P, Chetta A, de Blasio F, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36:1042–8.
Zanini A, Chetta A, Gumiero F, Della Patrona S, Casale S, Zampogna E, Aiello M, Spanevello A. Six-minute walking distance improvement after pulmonary rehabilitation is associated with baseline lung function in complex COPD patients: a retrospective study. Biomed Res Int. 2013;2013:1–6.
Ragaselvi S, Janmeja AK, Aggarwal D, Sidana A, Sood P. Predictors of response to pulmonary rehabilitation in stable chronic obstructive pulmonary disease patients: a prospective cohort study. J Postgrad Med. 2019;65:101–6.
Selzler A-M, Simmonds L, Rodgers WM, Wong EYL, Stickland MK. Pulmonary rehabilitation in chronic obstructive pulmonary disease: predictors of program completion and success. COPD J Chronic Obstr Pulm Dis. 2012;9:538–45.
Li C, Li X, Han H, Cui H, Wang G, Wang Z. Diaphragmatic ultrasonography for predicting ventilator weaning: a meta-analysis. Medicine (Baltimore). 2018;97:e10968.
Rabinovich RA, Ardite E, Troosters T, Carbo N, Alonso J, Gonzalezde Suso JM, Vilaro J, Barbera JA, Polo MF, Argiles JM, et al. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1114–8.
Lewinska A, Shahnazaryan K. The use of diaphragm ultrasonography in pulmonary physiotherapy of COPD patients: a literature review. J Clin Med 2020; 9.
Gibson GJ, Whitelaw W, Siafakas N, Supinski GS, Fitting JW, Bellemare F, Loring SH, Troyer AD, Grassino AE. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624.
Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD. Monitoring recovery from diaphragm paralysis with ultrasound. Chest. 2008;133:737–43.
McKenzie DK, Butler JE, Gandevia SC. Respiratory muscle function and activation in chronic obstructive pulmonary disease. J Appl Physiol. 2009;107:621–9.
Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, Dubé BP, Fauroux B, Gea J, Guenette JA, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J 2019; 53.
Bhatt SP, Luqman-Arafath TK, Gupta AK, Mohan A, Stoltzfus JC, Dey T, Nanda S, Guleria R. Volitional pursed lips breathing in patients with stable chronic obstructive pulmonary disease improves exercise capacity. Chron Respir Dis. 2013;10:5–10.
Corbellini C, Boussuges A, Villafane JH, Zocchi L. Diaphragmatic mobility loss in subjects with moderate to very severe COPD may improve after in-patient pulmonary rehabilitation. Respir Care. 2018;63:1271–80.
Acknowledgements
Not applicable.
Funding
This work was supported by Grants-in-Aid for Scientific Research (21K11325).
Author information
Authors and Affiliations
Contributions
MS, YH, and YC made substantial contributions to the conception and design of the work. MS, YH, and RS made substantial contributions to the data acquisition. MS and HM made substantial contributions to the analysis. All of the listed authors designed the study and were involved in the interpretation of the data. MS and HM drafted the work. YH, MS, TK, YC, ON, KS, KF, YT, and HM revised the report critically for important intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kindai University School of Medicine (31-086). Written informed consent was obtained from all participants.
Consent for publication
If the manuscript is accepted, we approve it for publication in Respiratory Research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shiraishi, M., Higashimoto, Y., Sugiya, R. et al. Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Respir Res 22, 271 (2021). https://doi.org/10.1186/s12931-021-01870-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-021-01870-1