- Research
- Open access
- Published:
Real-life cost-effectiveness of benralizumab in patients with severe asthma
Respiratory Research volume 22, Article number: 163 (2021)
Abstract
Background
Availability of clinically effective and cost-effective treatments for severe asthma would be beneficial to patients and national healthcare systems. The aim of this study was to evaluate clinical outcomes and healthcare expenditure after incorporating benralizumab into the standard treatment of refractory eosinophilic asthma.
Methods
This was a cross-sectional multicentre study of consecutive patients with refractory eosinophilic asthma who received treatment with benralizumab during at least 12 months. Patient follow-up was performed in specialised severe asthma units. The main effectiveness parameters measured were: the avoidance of one asthma exacerbation, a 3-point increase in the asthma control test (ACT) score, and the difference in utility scores (health-related quality of life) between a 1-year baseline treatment and 1-year benralizumab treatment. The health economic evaluation included direct costs and incremental cost-effectiveness ratios (ICERs).
Results
After 1 year of treatment with benralizumab, patients with refractory eosinophilic asthma showed an improvement in all the effectiveness parameters analysed: improvement of asthma control and lung function, and decrease in the number of exacerbations, oral corticosteroid (both as corticosteroid courses and maintenance therapy), and inhaled corticosteroid use. The total annual cost per patient for the baseline and benralizumab treatment periods were €11,544 and €14,043, respectively, reflecting an increase in costs due to the price of the biological agent but a decrease in costs for the remaining parameters. The ICER was €602 per avoided exacerbation and €983.86 for every 3-point increase in the ACT score.
Conclusions
All the pharmacoeconomic parameters analysed show that treatment with benralizumab is a cost-effective option as an add-on therapy in patients with refractory eosinophilic asthma.
Background
Asthma is a heterogeneous condition characterised by chronic inflammation of the pulmonary airways [1] with an estimated 300 million people currently affected worldwide [2], and its prevalence has been increasing in recent years. Severe asthma is defined as asthma that requires maximal, optimised inhaled corticosteroid (ICS) therapy plus another controller treatment to remain under control, or asthma that is uncontrolled despite this therapy [1, 3]. It is estimated to affect 5–10% of the asthma population. Refractory eosinophilic asthma is a phenotype of severe asthma characterised primarily by increased blood eosinophils and frequent exacerbations despite corticosteroid therapy [1, 3]. Several studies have shown that despite the availability of effective treatments such as ICS, long-acting β2-agonists (LABA), tiotropium, and leukotriene modifiers, over 50% of asthma patients are assessed as not well-controlled in standard clinical practice [4,5,6] and many require further therapies such as oral corticosteroids (OCS) and biologics [7]. Over 50% of deaths caused by asthma are reported in patients with a history of severe asthma [8] and this severe condition is associated with increased morbidity, healthcare costs, and mortality [9, 10].
Availability of clinically effective and cost-effective treatments for severe asthma would be beneficial to patients and national healthcare systems. The use of biological therapies could lead to improved clinical outcomes in severe asthma together with a reduced economic burden of the disease. There are currently four biologic agents available in Spain for the treatment of severe asthma: omalizumab, mepolizumab, reslizumab, and benralizumab. Several studies performed in different healthcare settings have provided evidence on the advantages of omalizumab [11,12,13], mepolizumab [14, 15], and reslizumab [16] as add-on therapies in terms of cost-effectiveness in the management of severe asthma patients.
Benralizumab (Fasenra®, AstraZeneca) binds to the human IL-5 receptor (IL-5R) through its Fab domain, thereby preventing IL-5 from binding to its receptor and inhibiting differentiation and maturation of eosinophils in the bone marrow. In addition, this antibody has the ability to bind through its afucosylated Fc domain to the RIIIa region of the Fc receptor on natural killer cells, macrophages, and neutrophils, thereby enhancing antibody-dependent cell-mediated cytotoxicity of both blood eosinophils and tissue-resident eosinophils [17, 18]. In three phase 3 clinical trials (SIROCCO [19], CALIMA [20], and ZONDA [21]), the administration of 30 mg subcutaneous benralizumab every 8 weeks (every 4 weeks for the first three doses) reduced the annual rate of severe asthma exacerbations and the use of OCS, and improved symptom control and lung function determined by the forced expiratory volume in 1 s (FEV1). Additionally, the BORA study [22] has shown its long-term efficacy and safety. Despite having been approved only recently, other studies are confirming these good results also in real life [23,24,25]. To date, however, no real-life pharmacoeconomics studies of benralizumab have been conducted. The purpose of this study was therefore to assess the cost-effectiveness of benralizumab therapy and its 1-year effectiveness, based on the decrease in the number of exacerbations and OCS use, and the improvement of asthma control and lung function in the real world.
Methods
Study population and study design
This multicentre study included 44 patients with refractory eosinophilic asthma who received treatment with benralizumab for at least 12 months at the Asthma Units of Hospital Costa del Sol (Marbella, Spain) and Hospital Virgen de la Victoria (Málaga, Spain). All patients were diagnosed with asthma based on objective tests (FEV1 reversibility ≥ 12%, positive results to methacholine, or FEV1 variability ≥ 20%).
A standardised protocol was used to try to improve these patients’ asthma control. This consisted of ensuring adherence to therapy and appropriate inhaler use, providing health education, adjusting treatment, and ruling out comorbidities [26,27,28].
Benralizumab treatment initiation criteria were as follows:
-
18-year-old patient or older with refractory eosinophilic asthma [3];
-
GINA guidelines step 5 [1];
-
2 or more exacerbations during the previous year with use of OCS despite receiving appropriate treatment for the degree of severity or corticosteroid dependence;
-
Presence of eosinophilic inflammation: eosinophil count ≥ 300 cells/µL in peripheral blood during the previous 12 months or ≥ 150 cells/µL in case of corticosteroid dependence.
All patients were treated with benralizumab for at least 12 months and were included in the analysis.
Patients previously treated with another biologic agent but who had failed to respond based on the physician’s judgement were included. The following criteria for lack of response to a prior biologic treatment were applied:
-
Continued use of maintenance OCS despite receiving biologic therapy for at least 12 months, or
-
Less than a 50% reduction in exacerbations after at least 12 months of biologic therapy.
At least three visits were performed after treatment initiation with benralizumab: one at 3 months of treatment, one at 6 months of treatment, and a final visit at 12 months of benralizumab treatment.
Written informed consent was obtained from all participants. The study was reviewed by the Spanish Medicines and Health Products Agency and approved by the ethics committee Comité de ética provincial de Málaga.
Clinical, analytical, and lung function variables
A database was compiled from complete medical records, with data from diagnosis to study enrolment. A standardised protocol was applied for the prospective collection of sociodemographic data (sex, age), clinical profile (age at diagnosis of asthma, smoking, atopy, presence of nasal polyps), exacerbations, use of corticosteroid therapy, and basic blood test. Dyspnoea was evaluated by means of the modified Medical Research Council Scale for Dyspnoea [29], and we divided patients into two stage groups (0–2 and 3–4) according to their degree of dyspnoea. We used the asthma control test (ACT) to evaluate the degree of asthma control in the 4 weeks prior to the clinical interview. The ACT [30] is a self-administered tool that is easy for patients to complete. It includes four symptom-relief questions plus a patient’s self-assessment of asthma control [1] in the last 4 weeks, with scores ranging from 5 (poor control) to 25 (complete control), and has been validated in Spanish [31]. A 3-point difference in the ACT score has been estimated as a minimally important difference [32]. Nasal polyposis, a frequent comorbidity in severe asthma, was diagnosed by an otorhinolaryngologist by direct visualisation of the polyps with endoscopic examination. Patients were considered as atopic when they had positive allergic prick tests or positive specific IgE to the most prevalent pneumo-allergens in our area, provided that these positive findings also had clinical relevance. Corticosteroid dependence was defined as the daily use of OCS during at least 6 months. Lastly, severe asthma exacerbations, defined as exacerbations requiring the use of systemic corticosteroids for at least 3 days and/or leading to an emergency department visit and/or hospital admission, were studied [33].
All patients were trained to identify exacerbation symptoms. They were also asked to record detailed information about their condition and their prescriptions (systemic steroids). This information was verified in their medical records.
Fractional exhaled nitric oxide (FeNO) was measured with a conventional chemiluminescence analyser (NIOX, Aerocrine AB, Sweden) using the online standardised single-breath technique, and was followed by the performance of a spirometry. Both procedures conformed to international guidelines [34, 35].
Patients were classified according to their response at 12 months of benralizumab treatment as patients with complete response, patients with controlled asthma, patients with partial response, and patients with no response, based on the Spanish consensus for severe asthma in adults [36]. Response criteria are shown in Table 1.
All variables were measured during the baseline visit and at 3, 6 and 12 months of treatment.
Outcomes
Three different measures of effectiveness were considered for the pharmacoeconomics analysis:
-
1.
Avoidance of one asthma exacerbation [33],
-
2.
3-point increase in the ACT score [32],
-
3.
Difference in utility (health-related quality of life) between baseline treatment and benralizumab treatment (EQ-5D index obtained from Spanish asthmatic patients [37]). To calculate utility values, we used the benefits obtained in Spain according to the ACT score in patients with severe asthma [37]. Utility values were as follows: 0.91 for patients with controlled asthma and 0.73 for patients with uncontrolled asthma.
Other parameters of effectiveness such as reduction in severe exacerbations and emergency department visits, reduction in the use of oral and inhaled steroids, and improvement of lung function and asthma control based on the ACT score were measured.
Cost analysis
Direct healthcare costs of these patients’ management during the year prior to the start of benralizumab were determined and compared with the direct healthcare costs at 1 year on benralizumab treatment. Direct costs included pharmacological costs and use of healthcare resources. Pharmacological costs included medications for the management of severe asthma, such as ICS, OCS, and monoclonal antibodies (omalizumab, mepolizumab, reslizumab, and benralizumab). Healthcare resources considered were: tests (spirometry, bronchodilator test, FeNO, skin prick, haemogram, biochemistry, and chest CT) carried out at visits to the asthma unit during the year prior to the start of benralizumab treatment and the year on benralizumab treatment, hospital admissions due to exacerbations, recorded number of emergency department visits (primary care emergency visits and hospital emergency visits), and prednisone courses administered during exacerbations.
The costs of the healthcare resources used were obtained from the Andalusian Healthcare Service [38] and the costs of the medicines used were obtained from the Spanish Ministry of Health and from the Summary of Product Characteristics of each product [39, 40]. Costs were determined and expressed in 2020 Euros (€) (Table 2).
Pharmacoeconomics analysis
Cost-effectiveness analyses (CEAs)—defined in a broad sense, i.e., including cost utility analyses (CUAs)—are faced with the difficulty of having to use different units for measuring costs and health outcomes, as costs are expressed in monetary units and health outcomes are measured in physical/clinical units or quality-adjusted life-years (QALYs). Therefore, a programme cannot be accepted or rejected in absolute terms, but only in relation to another programme which acts as a term of comparison, reference, or control.
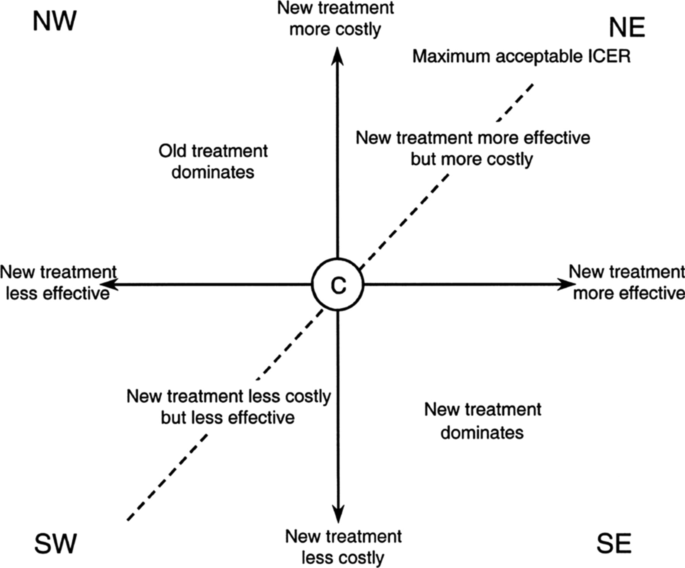
All possible cost-effectiveness comparisons can be represented graphically on the so-called cost-effectiveness plane or space (Fig. 1), with differences in effectiveness plotted along the X-axis and differences in costs plotted along the Y-axis. Thus, the quadrants of the cost-effectiveness plane indicate four possible situations. The figure clearly illustrates that decision rules are required only for the cost-effectiveness pairs situated in the NE and SW quadrants.
Therefore, decision rules in CEAs -and CUAs- are based on the comparison of cost increases (∆C) with effectiveness increases (∆E) to calculate the extra cost per additional unit of effectiveness of the programme being evaluated in relation to the reference programme. The incremental cost-effectiveness ratio (ICER) was used to compare the impact of benralizumab treatment in terms of costs and clinical outcomes with the baseline treatment both over a 1-year period, and was calculated as follows:
Sensitivity analysis
A probabilistic sensitivity analysis was carried out using a first-order Monte Carlo simulation in a hypothetical cohort of 10,000 patients for both costs and effectiveness for the two regimens under study: baseline regimen (pre-benralizumab) versus new regimen (benralizumab).
Our pharmacoeconomics analysis also took into account the net benefit obtained both in monetary and nonmonetary (health outcomes) terms.
The Net Monetary Benefit (NMB) is a summary statistic that represents the value of an intervention in monetary terms when the willingness to pay (WTP) threshold for a benefit unit (e.g., a health outcome measure or QALY) is known. The NMB is calculated as follows:
where E = effectiveness; WTP = willingness-to-pay threshold; C = cost.
In Spain, the WTP threshold varies between €22,000 and €24,000 per year [41].
The Net Health Benefit (NHB) was also used. This is a summary statistic representing the impact of a new intervention on the health of the population. The NHB assumes that “lost health” can be estimated as an “opportunity cost” to represent health lost as a result of the displacement of resources to fund a new intervention. NHB is normally measured using QALYs and calculated as: incremental benefit − (incremental cost/WTP threshold). Given a certain WTP, a positive NHB implies that the general health of the population would increase as a result of the new intervention, while a negative NHB implies that the health benefits of the new intervention are not sufficient to compensate for the health losses arising from health care that is no longer funded to finance the new treatment.
Statistical analysis
All the data were analysed using the software SPSS v25 licensed from the University of Malaga. A descriptive analysis was performed using measures of central tendency, position, and dispersion for quantitative variables, and frequency distribution for qualitative variables. To assess changes at 3, 6, and 12 months compared with baseline, Student’s t test for paired samples was used (Wilcoxon rank test for non-normal distributions) and McNemar test was used for qualitative variables. A generalised linear model was used for the analysis of repeated measures with four assessment timepoints.
Effect-size calculations were performed to differentiate between statistically significant and clinically relevant results [41, 42]. By using Cohen’s “d” index we can determine the degree of association between two variables or their differences. Cohen’s “d” index allows to quantify the effect of treatments in relation to the clinical criterion analysed, and effect sizes can be classified as: insignificant effect (− 0.15 and < 0.15); small effect (≥ 0.15 and < 0.40); medium effect (≥ 0.40 and < 0.75); large effect (≥ 0.75 and < 1.10); very large effect (≥ 1.10 and < 1.45); enormous effect > 1.45 [43, 44].
Confidence intervals (CIs) for the ICERs were computed based on bootstrapping, using sampling with replacement (the size of the new samples was equal to the original size). We ran 10,000 Monte Carlo simulations, and the 2.5th and 97.5th percentiles from the simulations were used to determine a 95% CI. Statistical significance was set at p < 0.05.
Results
Patient population
A total of 44 patients with refractory eosinophilic asthma who received benralizumab treatment during at least 12 months were enrolled. Clinical characteristics of the study population are shown in Table 3. In short, most patients were women in their fifth decade of life, overweight, and with significant eosinophilic inflammation as evidenced by the presence of eosinophilia. In addition, all patients were receiving high dose ICS, LABA, and at least one other controller.
Parameters assessed
Clinical, functional, and laboratory data at baseline and at 3, 6, and 12 months of treatment as well as the comparison between values at baseline and at 12 months are presented in Table 4 and Fig. 2.
Clinical, functional, and laboratory data at baseline and at 3, 6, and 12 months of treatment. a FEV1 mL; b FEV1%; c ACT (asthma control test); d No. of emergency department visits: e No. of oral corticosteroid courses; f Oral prednisone dose (mg/day); g Inhaled budesonide dose (μg/day); h Blood eosinophils (cells/μL). Data expressed as means. *p < 0.001
At 1 year of treatment, there was an 83% reduction in emergency department visits, an 88% reduction in severe exacerbations, a 79.8% reduction in the prednisone (or equivalent) dose, a 55.6% reduction in the number of corticosteroid-dependent patients, and an 82.8% reduction in the number of OCS courses. 65.9% of patients had required zero emergency department visits during the 1-year treatment with benralizumab and 47.7% consumed zero OCSs (both as corticosteroid courses and maintenance therapy) during that period.
We classified patients according to their response at 12 months of benralizumab treatment based on the Spanish Severe Asthma Consensus [36]. Results are shown in Fig. 3. We found that 100% of patients responded to benralizumab treatment, and 79.6% had a very good response (controlled asthma or complete response), while only nine patients showed a partial response with eight remaining corticosteroid-dependent (although with a reduction in OCS ≥ 50%) and one, who was corticosteroid-dependent and despite managing to discontinue permanently OCS, required two courses of OCS during that year, although a > 50% reduction in OCS use was observed. Of these nine patients with a partial response, six had had their asthma previously treated with a biological agent. No patients were classified as non-responders.
Among the side effects experienced by nine patients (20.5%), the most common ones were arthralgias, headaches, and dysthermia. However, all side effects were mild and there were no treatment discontinuations due to side effects.
Direct healthcare costs
Table 5 compares the cost of healthcare resources used in the preceding year and in the year with benralizumab therapy. Costs increased during the year following benralizumab treatment initiation due to the price of the biological treatment, but the costs of complementary tests, emergency care and admissions, and oral and inhaled corticosteroids decreased.
Cost-effectiveness analysis (CEA)
Decrease in the number of exacerbations
Table 6 shows the results of the CEA based on the reduction of severe exacerbations. As shown, an incremental cost of €602/year is required to avoid one severe exacerbation, and €3300/year to avoid any exacerbation in a given patient. On the other hand, the number of severe exacerbations correlates (Pearson’s R coefficient) with the cost of OCS (R = 0.839; 95% CI 0.709–0.913) and with the cost of emergency department visits (R = 0.849; 95% CI 0.726–0.919).
The Fig. 4 shows the incremental cost-effectiveness of the new treatment with benralizumab compared with the previously used treatment option (baseline situation) in patients with refractory eosinophilic asthma. Values shown indicate the uncertainty surrounding the incremental cost-effectiveness ratio. Each blue dot thus represents a patient (one cost, one effectiveness). The Monte Carlo simulation extrapolates the results to a hypothetical cohort of 1000 patients. Any point below the orange line (patient with its cost and effectiveness) indicates that the procedure is efficient in our country.
Asthma control test
Table 7 shows the results of the estimated cost (in Euros/year) to achieve a 1-point ACT increase and a 3-point increase, and to achieve an ACT score > 20 (starting from an ACT score of 13 points, the baseline mean). It also provides a cost-effectiveness analysis expressed as a function of the percentage of effectiveness achieved by the increase of the ACT score.
Figure 5 shows the incremental cost-effectiveness for benralizumab versus baseline 1-year treatment periods, using a Monte Carlo simulation of 10,000 patients.
Cost-utility analysis (CUA)
The utility gained at 1 year of benralizumab treatment versus the baseline treatment period was 0.138 QALYs (incremental utility) while the incremental cost was €2499. The Table 8 shows the cost-utility analysis carried out. The incremental cost-utility ratio (ICUR) obtained was €18,177/QALY.
Figure 6 shows the incremental cost-utility of benralizumab versus baseline treatment periods (other biological treatments) at 1 year of treatment, using a Monte Carlo simulation of 10,000 patients. Values shown indicate the uncertainty surrounding the ICUR. For a WTP of €24,000, the likelihood of benralizumab providing a better cost-utility compared with the baseline treatment option was 80.9%.
Net benefit
The Net Monetary Benefit (NMB) obtained with benralizumab was €813 (NMB = [0.138 * 24,000] − 2499 = 813) which means that, as it is higher than 0, it is an efficient treatment option compared to the baseline option.
The positive NHB outcome suggests that it is a measure that improves the general health of the population (NHB = 0.138 − [2499/24,000] = 0.034).
Discussion
This was an effectiveness and pharmacoeconomics study on the use of benralizumab in real life in patients with refractory eosinophilic asthma.
Before prescribing a biological product, essential aspects such as a correct diagnosis, adherence to the prescribed therapeutic plan, and appropriate inhaler technique (if applicable) should be reviewed. Once they have been confirmed, therapy with biologics should be considered in case of a lack of clinical effectiveness [1]. In recent years, the health authorities have approved four new drugs classified as biologics, in addition to omalizumab: mepolizumab, benralizumab, reslizumab, and more recently dupilumab (although marketing approval for the latter has not been granted in Spain yet). However, little is known about their effectiveness in actual clinical practice, and even less is known about their cost-effectiveness in usual care settings. The main objective of the present study was to address these questions.
With regard to the effectiveness of a 1-year benralizumab treatment in real life, our findings have shown a significant reduction in the number of emergency department visits, severe exacerbations, and use of OCS as well as an improvement in asthma control and lung function, in line with the SIROCCO [19], CALIMA [20], and ZONDA [21] pivotal studies. Likewise, real-life data showing similar results after 6 months [23, 45] and 1 year of treatment [24, 25, 46] have been published. Our study revealed that 65.9% of patients had required zero emergency department visits during the 1-year treatment with benralizumab, a slightly better outcome than the 40% found by Kavanagh et al. [24], while a 55.6% reduction in the number of corticosteroid dependent patients was obtained, a value similar to the 51.4% described by these authors. We also observed a significant decrease in the number of OCS courses used. Recent studies have shown that OCS courses are associated with a greater probability of experiencing side effects [47] and therefore a reduction in the use of OCS may improve outcomes in patients with asthma.
Another interesting finding was the reduction in ICS which we had already observed in our previous real-life study with benralizumab during 6 months [23] and which has also been demonstrated in other real-life studies [24]. This, however, had not been shown in pivotal studies, and the randomized controlled SHAMAL study is currently under way to assess if the use of ICS may be decreased in these patients (ClinicalTrials.gov Identifier: NCT04159519).
The percentage of responders in our investigation (100%) was again higher than in Kavanagh et al.’s study [24] where 13.8% of patients did not respond. A universally accepted consensus to define a complete response or super response is lacking. The response criteria used in our study were those recommended by the Spanish Consensus on Severe Asthma [36], which are more restrictive than those described by Kavanagh et al. [24] (reduction of ≥ 50% in annualised exacerbation rate or in maintenance OCS dose after 48 weeks of treatment) as they include parameters such as asthma control and lung function. Thus, in our case, a patient with a complete response was defined as a patient with zero exacerbations during 1 year, an ACT score ≥ 20, an FEV1 ≥ 80%, and no maintenance OCS. Based on these criteria, only 12 patients (27.3%) showed a complete response. Kavanagh et al. [24] defined super responders as patients with zero exacerbations and no maintenance OCS for asthma, and 39% of their patients met these criteria. With these criteria, 59.1% of our patients would qualify as super responders. Our findings are better than those obtained in the pivotal studies SIROCCO [19], CALIMA [20], and ZONDA [21] and in certain real-life studies conducted [24], probably because of the significant eosinophilic inflammation experienced by our patients who had a mean blood eosinophil count of 718.3 ± 287.5 cells/μL.
With regard to safety, no serious side effects were found and no treatment was discontinued due to side effects. These results reinforce the data provided by the pivotal studies on benralizumab safety which showed low rates of discontinuation due to side effects (2% in SIROCCO [19] and CALIMA [20], and 2–3% in BORA [22]).
Pharmacoeconomics analysis
All the pharmacoeconomic parameters analysed show that treatment with benralizumab is a cost-effective option as an add-on therapy in patients with refractory eosinophilic asthma.
The total cost per patient during the preceding year was €11,544 whereas, during the subsequent year on treatment with benralizumab, this was €14,043. This annual increase of €2499 was the result of the price of the biological treatment and the decrease in costs related to hospital admissions, emergency department visits, and OCS or ICS use. To date, there has only been one study published analysing benralizumab cost-effectiveness based on pivotal studies [48], in which a total increase per patient and year was also found and was also the result of the increase in costs from the use of benralizumab. This has also been evidenced with other biological treatments for asthma as shown in several systematic reviews [49, 50] demonstrating that time horizon and drug price are among the key drivers of the incremental cost-effectiveness ratio.
Incremental costs of €602/year and €3300/year to avoid a severe exacerbation and any exacerbation, respectively, in a given patient were found. Furthermore, for a 1-point ACT score increase, an incremental cost of €327.95/year was found, and for a 3-point increase the incremental cost was €983.86/year. These costs are significantly lower compared with those found in other pharmacoeconomics studies for other asthma biologics such as omalizumab [11, 51] and mepolizumab [15]. This is most likely due to the fact that current prices are lower, to differences in healthcare systems, and to a greater effectiveness of benralizumab in our study resulting from the fact that patients were managed in specialised asthma units, which enabled us to apply strict inclusion criteria and enrol patients with very severe asthma and severe eosinophilic inflammation.
With effectiveness measured as a percentage of gain in ACT score and taking into account that an efficient alternative in Spain is considered when the cost-effectiveness threshold lies between €22,000 and €24,000 [52], the probability of benralizumab being a cost-effective option for a WTP of €24,000 was 99.8%, and it was found to be dominant (more effective and less costly) in almost 10% compared with the baseline alternative.
The NMB obtained was positive, indicating that benralizumab was a more efficient alternative than its comparator. Furthermore, the positive incremental NMB indicates that the intervention was cost-effective compared to the alternative regimen based on the WTP threshold in Spain (€22,000–€24,000). Thus, the cost of obtaining an additional benefit was below the maximum amount decision makers would be willing to pay for this benefit. Furthermore, the positive NHB outcome suggests that it is a measure that improves the general health of the population.
In addition, at a WTP threshold of €24,000, the probability of benralizumab having a better cost-utility for a WTP of €24,000 was 81% and was found to be dominant (more utilities and lower costs) in 8.3% compared with the baseline alternative.
Overall, based on our study, benralizumab in association with the standard treatment of refractory eosinophilic asthma appears to be a cost-effective option in Spain with a cost < €24,000/QALY gained, whereas in a prior cost-effectiveness study in Sweden based on pivotal studies [48] this cost ranged between €40,000 and €70,000/QALY gained. These considerably higher values are most likely due to differences in prices between the two countries and differences between both national healthcare systems. In fact, cost-effectiveness results for a particular drug may vary from country to country because of these differences. Thus, mepolizumab was reported to be cost-effective for the treatment of severe eosinophilic asthma in the United Kingdom [53] but was found not to be cost-effective for similar patients in the United States at commonly cited WTP thresholds [15].
Limitations
Our study has some limitations. Because this was a real-life study, there was no placebo control group and therefore a placebo effect could not be assessed. Additionally, as a result of the pandemic caused by SARS-CoV-2, spirometry at 1 year of treatment with benralizumab could not be performed in four of 44 patients. This explains why figures in Tables 1 and 2 are different. Likewise, FeNO measurements could not be assessed in these patients and were excluded from the analysis.
Its strengths, on the other hand, lie in the fact that, to our knowledge, this is the only study of benralizumab with real life data to date. In addition, it is a multicentre study that was conducted at two different severe asthma units with a broad experience in the management and treatment of this disease. Finally, this was an independent study with no external funding and without the involvement of any pharmaceutical company.
Conclusions
We observed a clear improvement in asthma control and lung function as well as a reduction in severe exacerbations, emergency department visits, use of OCS (both as corticosteroid courses and maintenance therapy), and ICS in patients with refractory eosinophilic asthma treated with benralizumab for 1 year. Moreover, benralizumab appears to be a cost-effective option (less than €24,000/QALY) as an add-on treatment in patients with refractory eosinophilic asthma in Spain.
Availability of data and materials
The data sets analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ACT:
-
Asthma control test
- AERD:
-
Aspirin-exacerbated respiratory disease
- BD:
-
Bronchodilator
- BMI:
-
Body mass index
- FeNO:
-
Fractional exhaled nitric oxide
- FEV1:
-
Forced expiratory volume in 1 s
- GINA:
-
Global Initiative for Asthma
- ICER:
-
Incremental cost-effectiveness ratio
- ICS:
-
Inhaled corticosteroids
- IL-5:
-
Interleukin-5
- IL-5R:
-
Interleukin-5 receptor
- IR:
-
Interquartile range
- LABA:
-
Long-acting β2-agonists
- OCS:
-
Oral corticosteroids
References
Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA). 2020. http://www.ginasthma.org/.
Bousquet J, Clark TJH, Hurd S, Khaltaev N, Lenfant C, O’byrne P, et al. GINA guidelines on asthma and beyond. Allergy. 2007;62(2):102–12.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73.
Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program: the global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–78.
Demoly P, Gueron B, Annunziata K, Adamek L, Walters RD. Update on asthma control in five European countries: results of a 2008 survey. Eur Respir Rev. 2010;19:150–7.
González Barcala FJ, de la Fuente-Cid R, Alvarez-Gil R, Tafalla M, Nuevo J, Caamaño-Isorna F. Factors associated with asthma control in primary care patients: the CHAS study. Arch Bronconeumol. 2010;46(7):358–63.
Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902.
Levy ML. The national review of asthma deaths: what did we learn and what needs to change? Breathe (Sheff). 2015;11(1):14–24.
Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139–51.
Husereau D, Goodfield J, Leigh R, Borrelli R, Cloutier M, Gendron A. Severe, eosinophilic asthma in primary care in Canada: a longitudinal study of the clinical burden and economic impact based on linked electronic medical record data. Allergy Asthma Clin Immunol. 2018;14:15.
Entrenas Costa LM, Casas-Maldonado F, Soto Campos JG, Padilla-Galo A, Levy A, Álvarez Gutiérrez FJ, et al. Economic impact and clinical outcomes of omalizumab add-on therapy for patients with severe persistent asthma: a real-world study. Pharmacoecon Open. 2019;3(3):333–42.
Levy AN, Ruiz GAAJ, Garcia-Agua Soler N, Sanjuan MV. Cost-effectiveness of omalizumab in severe persistent asthma in Spain: a real-life perspective. J Asthma. 2015;52(2):205–10.
Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess. 2013;17(52):1–342.
Llanos JP, Ortega H, Bogart M, Packnett ER, Manjelievskaia J, Bell CF, Hahn B. Real-world effectiveness of mepolizumab in patients with severe asthma: an examination of exacerbations and costs. J Asthma Allergy. 2020;13:77–87.
Whittington MD, McQueen RB, Ollendorf DA, Tice JA, Chapman RH, Pearson SD, Campbell JD. Assessing the value of mepolizumab for severe eosinophilic asthma: a cost-effectiveness analysis. Ann Allergy Asthma Immunol. 2017;118(2):220–5.
Lam J, Hay JW, Salcedo J, Kenyon NJ. A cost-effectiveness analysis of reslizumab in the treatment of poorly controlled eosinophilic asthma. J Asthma. 2019;56(8):872–81.
Fasenra [US prescribing information]. AstraZeneca Pharmaceuticals LP. 2017.
Dávila González I, Moreno Benítez F, Quirce S. Benralizumab: a new approach for the treatment of severe eosinophilic asthma. J Investig Allergol Clin Immunol. 2019;29(2):84–93.
Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–27.
FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–41.
Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–58.
Busse WW, Bleecker ER, FitzGerald JM, Ferguson GT, Barker P, Sproule S, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7:46–59.
Padilla-Galo A, Levy-Abitbol R, Olveira C, Valencia Azcona B, Pérez Morales M, Rivas-Ruiz F, et al. Real-life experience with benralizumab during 6 months. BMC Pulm Med. 2020;20(1):184.
Kavanagh JE, Hearn AP, Dhariwal J, d’Ancona G, Douiri A, Roxas C, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2021;159(2):496–506.
Miralles López JC, Escudero Pastor AI, Carbonell Martínez A, Navarro Garrido C, Bonilla Pacheco Y, Petrik Petrik Y. Benralizumab in real life. J Investig Allergol Clin Immunol. 2020. https://doi.org/10.18176/jiaci.0599 (Online ahead of print).
Padilla-Galo A, Olveira C, Fernández de Rota-Garcia L, Marco-Galve I, Plata AJ, Alvarez A, et al. Factors associated with bronchiectasis in patients with uncontrolled asthma; the NOPES score: a study in 398 patients. Respir Res. 2018;19(1):43.
Fernandes JC, Biskupiak WW, Brokaw SM, Carpenedo D, Loveland KM, Tysk S, et al. Outcomes of the montana asthma home visiting program: a home-based asthma education program. J Asthma. 2019;56(1):104–10.
Rodríguez-García C, Lourido-Cebreiro T, González-Barcala FJ. The ATAUD Study: the need to improve adherence. Arch Bronconeumol. 2019;55(10):509–10.
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–6.
Natham RA, Sorkness ChA, Kosinski M, Schatz M, Li J, Marcus PH. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65.
Vega JM, Badia X, Badiola C, López-Viña A, Olaguíbel JM, Picado C. Validation of the Spanish version of the asthma control test (ACT). J Asthma. 2007;44:867–72.
Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719–23.
Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99.
American Thoracic Society. Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children. Am J Respir Crit Care Med. 2005;171(8):912–30.
Standardization of spirometry. Statement of the American thoracic Society. Am Rev Respir Dis. 1987;136(5):1285–98.
Álvarez-Gutiérrez FJ, Blanco-Aparicio M, Plaza V, Cisneros C, García-Rivero JL, Padilla A, et al. Documento de consenso de asma grave en adultos. Actualización 2020. Open Respir Arch. 2020;2(3):158–74.
Gimena H, Pont À, Garin O, Martí M, Alonso J, Ganse E, The ASTRO-LAB Group, et al. Validity of the five-level new version of the EQ-5D in asthma patients. J Thorac Dis. 2016;8(Suppl 5):AB022. https://doi.org/10.21037/jtd.2016.s022.
Precios públicos Servicio Andaluz de Salud. 2020. https://www.sspa.juntadeandalucia.es/servicioandaluzdesalud/profesionales/recursos-para-profesionales/precios-públicos. Accessed 23 Nov 2020.
SNS: Información sobre los productos incluidos en la prestación farmacéutica del SNS. Ministerio de Sanidad, Consumo y Bienestar Social. https://www.mscbs.gob.es/profesionales/nomenclator.do. Accessed 23 Nov 2020.
Fichas técnicas de producto. Ministerio de Sanidad, Consumo y Bienestar Social. https://cima.aemps.es/cima/. Accessed 23 Nov 2020.
Vallejo L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for Spanish NHS. Health Econ. 2018;27:746–61.
Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis. 1987;40:171–8.
Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27(Suppl 3):178–89.
Thalheimer W, Cook S. How to calculate effect sizes from published research articles: a simplified methodology. 2002, August. http://work-learning.com/effect_sizes.htm. Accessed 31 Nov 2002.
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Elrbaum Associates; 1988.
Menzella F, Ruggiero P, Galeone C, Scelfo C, Bagnasco D, Facciolongo N. Significant improvement in lung function and asthma control after benralizumab treatment for severe refractory eosinophilic asthma. Pulm Pharmacol Ther. 2020;64:101966.
Matsuno O, Minamoto S. Rapid effect of benralizumab for severe asthma with chronic rhinosinusitis with nasal polyps. Pulm Pharmacol Ther. 2020;64:101965.
Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–6.
Andersson M, Janson C, Kristensen T, Szende A, Golam S. Cost effectiveness of benralizumab for severe, uncontrolled oral corticosteroid-dependent asthma in Sweden. J Med Econ. 2020;23(8):877–84.
Rodriguez-Martinez CE, Sossa-Briceño MP, Castro-Rodriguez JA. Cost effectiveness of pharmacological treatments for asthma: a systematic review. Pharmacoeconomics. 2018;36:1165–200.
McQueen RB, Sheehan DN, Whittington MD, van Boven JFM, Campbell JD. Cost-effectiveness of biological asthma treatments: a systematic review and recommendations for future economic evaluations. Pharmacoeconomics. 2018;36:957–71.
Vennera Mdel C, Valero A, Uría E, Forné C, Picado C. Cost-effectiveness analysis of omalizumab for the treatment of severe persistent asthma in real clinical practice in Spain. Clin Drug Investig. 2016;36(7):567–78.
Bermejo I, Stevenson M, Cooper K, Harnan S, Hamilton J, Clowes M, et al. Mepolizumab for treating severe eosinophilic asthma: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36:131–44.
Acknowledgements
We thank the research team at the Costa del Sol Hospital for their support.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
APG and ALN had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. APG, ALN, and AJGR contributed substantially to the study design, data collection, analysis and interpretation, and the writing of the manuscript. RCLA, CO, BVA, MPM, NGAS, BTG, and IMC contributed substantially to the study design, data collection, and review of the manuscript. FR contributed to the study design, analysis, and interpretation of the data and review of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from all participants. The study was reviewed by the Spanish Medicines and Health Products Agency and approved by the ethics committee Comité de ética provincial de Málaga (Approval: SNH-BEN-2020-01).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: APG reports personal fees and non-financial support from NOVARTIS, personal fees from ASTRA-ZENECA, personal fees and non-financial support from GSK, and personal fees from TEVA. CO reports non-financial support from NOVARTIS and personal fees and non-financial support from TEVA. ALN reports personal fees and non-financial support from NOVARTIS, personal fees from ASTRA-ZENECA, personal fees and non-financial support from GSK, and personal fees from TEVA.
Other authors have no relevant COIs to disclose outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Padilla-Galo, A., García-Ruiz, A.J., Levy Abitbol, R.C. et al. Real-life cost-effectiveness of benralizumab in patients with severe asthma. Respir Res 22, 163 (2021). https://doi.org/10.1186/s12931-021-01758-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-021-01758-0